Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1
- PMID: 21536946
- PMCID: PMC3114386
- DOI: 10.2337/db10-1631
Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1
Erratum in
- Diabetes. 2011 Nov;60(11):3097
Abstract
Objective: Congenital hyperinsulinemic hypoglycemia is a group of genetic disorders of insulin secretion most commonly associated with inactivating mutations of the β-cell ATP-sensitive K(+) channel (K(ATP) channel) genes ABCC8 (SUR1) and KCNJ11 (Kir6.2). Recessive mutations of these genes cause hyperinsulinism that is unresponsive to treatment with diazoxide, a channel agonist. Dominant K(ATP) mutations have been associated with diazoxide-responsive disease. We hypothesized that some medically uncontrollable cases with only one K(ATP) mutation might have dominant, diazoxide-unresponsive disease.
Research design and methods: Mutations of the K(ATP) genes were identified by sequencing genomic DNA. Effects of mutations on K(ATP) channel function in vitro were studied by expression in COSm6 cells.
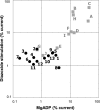
Results: In 15 families with diazoxide-unresponsive diffuse hyperinsulism, we found 17 patients with a monoallelic missense mutation of SUR1. Nine probands had de novo mutations, two had an affected sibling or parent, and four had an asymptomatic carrier parent. Of the 13 different mutations, 12 were novel. Expression of mutations revealed normal trafficking of channels but severely impaired responses to diazoxide or MgADP. Responses were significantly lower compared with nine SUR1 mutations associated with dominant, diazoxide-responsive hyperinsulinism.
Conclusions: These results demonstrate that some dominant mutations of SUR1 can cause diazoxide-unresponsive hyperinsulinism. In vitro expression studies may be helpful in distinguishing such mutations from dominant mutations of SUR1 associated with diazoxide-responsive disease.
Figures



References
-
- De León DD, Stanley CA. Mechanisms of disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab 2007;3:57–68 - PubMed
-
- Nestorowicz A, Wilson BA, Schoor KP, et al. Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum Mol Genet 1996;5:1813–1822 - PubMed
-
- Nestorowicz A, Inagaki N, Gonoi T, et al. A nonsense mutation in the inward rectifier potassium channel gene, Kir6.2, is associated with familial hyperinsulinism. Diabetes 1997;46:1743–1748 - PubMed

