Pharmacokinetics of lopinavir in HIV-infected adults receiving rifampin with adjusted doses of lopinavir-ritonavir tablets
- PMID: 21537021
- PMCID: PMC3122443
- DOI: 10.1128/AAC.01598-10
Pharmacokinetics of lopinavir in HIV-infected adults receiving rifampin with adjusted doses of lopinavir-ritonavir tablets
Abstract
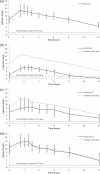
Rifampin coadministration dramatically reduces plasma lopinavir (LPV) concentrations. In healthy volunteers, doubling the dose of a lopinavir-ritonavir (LPV/r) capsule formulation overcame this interaction, but a subsequent study of double doses of the tablet formulation was stopped early owing to hepatotoxicity. However, healthy-volunteer study findings may not apply to HIV-infected adults. We evaluated the steady-state pharmacokinetics of LPV in HIV-infected adults virologically suppressed on an LPV/r regimen who were given rifampin, and the dose of the LPV/r tablet formulation was gradually increased. The steady-state pharmacokinetics of LPV/r were evaluated at baseline, a week after commencing rifampin, a week after the LPV/r dose was increased 1.5 times, and a week after the LPV/r dose was doubled. Twenty-one participants were enrolled. The median [interquartile range (IQR)] predose LPV concentrations (C(0)) were 8.1 (6.2 to 9.8) mg/liter at baseline, 1.7 (0.3 to 3.0) mg/liter after 7 days of rifampin, 5.9 (2.1 to 9.9) mg/liter with 1.5 times the dose of LPV/r, and 10.8 (7.0 to 13.1) mg/liter with double-dose LPV/r. There were no significant differences in the LPV area under the plasma concentration-time curve from 0 to 12 h (AUC(0-12)), C(0), C(12), maximum concentration of drug in serum (C(max)), or half-life (t(1/2)) between the baseline and double-dose LPV/r time points. Treatment was generally well tolerated, with two participants developing asymptomatic grade 3/4 transaminitis. Doubling the dose of the tablet formulation of LPV/r overcomes induction by rifampin. Less hepatotoxicity occurred in our cohort of HIV-infected participants than was reported in healthy-volunteer studies.
Figures
Comment in
-
HIV and TB coinfection: using adjusted doses of lopinavir/ritonavir with rifampin.Expert Rev Anti Infect Ther. 2011 Dec;9(12):1115-8. doi: 10.1586/eri.11.143. Expert Rev Anti Infect Ther. 2011. PMID: 22114961
References
-
- Ananworanich J., et al. 2005. Pharmacokinetics and 24-week efficacy/safety of dual boosted saquinavir/lopinavir/ritonavir in nucleoside-pretreated children. Pediatr. Infect. Dis. J. 24:874–879 - PubMed
-
- Centers for Disease Control Prevention 2007. Managing drug interactions in the treatment of HIV-related tuberculosis. http://www.cdc.gov/tb/publications/guidelines/HIV_AIDS.htm Accessed 13 August 2010
-
- Division of AIDS, National Institute of Allergy Infectious Diseases, National Institutes of Health 2004. Division of AIDS table for grading the severity of adult and pediatric adverse events. http://www.ucdmc.ucdavis.edu/clinicaltrials/documents/DAIDS_AE_GradingTa... Accessed 13 August 2010
-
- Gordin F. M., et al. 2004. Hepatotoxicity of rifampin and pyrazinamide in the treatment of latent tuberculosis infection in HIV-infected persons: is it different than in HIV-uninfected persons? Clin. Infect. Dis. 39:561–565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

