Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study
- PMID: 21537039
- PMCID: PMC3107744
- DOI: 10.1200/JCO.2010.32.6397
Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study
Abstract
Purpose: Bevacizumab, a recombinant humanized monoclonal antibody against vascular endothelial growth factor-A (VEGF-A), has clinical activity in multiple tumor types. We conducted a phase II trial to assess the activity and tolerability of single-agent bevacizumab in recurrent or persistent endometrial cancer (EMC).
Patients and methods: Eligible patients had persistent or recurrent EMC after receiving one to two prior cytotoxic regimens, measurable disease, and Gynecologic Oncology Group performance status of ≤ 2. Treatment consisted of bevacizumab 15 mg/kg intravenously every 3 weeks until disease progression or prohibitive toxicity. VEGF-A was assessed by immunohistochemistry in archival tumor and by enzyme-linked immunosorbent assay in pretreatment plasma. Primary end points were progression-free survival (PFS) at 6 months and overall response rate.
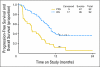
Results: Fifty-six patients were enrolled. Fifty-two patients were eligible and evaluable. Median age was 62 years, and prior treatment consisted of one or two regimens in 33 (63.5%) and 19 (36.5%) patients, respectively. Twenty-nine patients (55.8%) received prior radiation. Adverse events were consistent with those expected with bevacizumab treatment. No GI perforations or fistulae were seen. Seven patients (13.5%) experienced clinical responses (one complete response and six partial responses; median response duration, 6.0 months), and 21 patients (40.4%) survived progression free for at least 6 months. Median PFS and overall survival times were 4.2 and 10.5 months, respectively. Suggested associations were observed between high VEGF-A and adjusted hazard of death or tumor response when evaluated in tumor/plasma or plasma, respectively.
Conclusion: Bevacizumab is well tolerated and active based on PFS at 6 months in recurrent or persistent EMC and warrants further investigation.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2004;22:2159–2166. - PubMed
-
- Fiorica M, Brunetto VL, Hanjani P, et al. Phase II trial of alternating course of megestrol acetate and tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:10–14. - PubMed
-
- Whitney CW, Brunetto VL, Zaino RJ, et al. Phase II study of medroxyprogesterone acetate plus tamoxifen in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:4–9. - PubMed
-
- Lentz SS, Brady MF, Major FJ, et al. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 1996;14:357–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

