Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin
- PMID: 21537081
- PMCID: PMC3105371
- DOI: 10.1172/JCI43586
Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin
Abstract
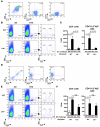
Patients with atopic dermatitis (AD) often suffer from food allergy and develop flares upon skin contact with food allergens. However, it is unclear whether T cells sensitized to allergens in the gut promote this skin inflammation. To address this question, we orally immunized WT mice and mice lacking the skin-homing chemokine receptor Ccr4 (Ccr4-/- mice) with OVA and then challenged them epicutaneously with antigen. Allergic skin inflammation developed in the WT mice but not in the mutants and was characterized by epidermal thickening, dermal infiltration by eosinophils and CD4+ T cells, and upregulation of Th2 cytokines. T cells purified from mesenteric lymph nodes (MLNs) of orally immunized WT mice transferred allergic skin inflammation to naive recipients cutaneously challenged with antigen, but this effect was lost in T cells purified from Ccr4-/- mice. In addition, the ability of adoptively transferred OVA-activated T cells to home to the skin following cutaneous OVA challenge was ablated in mice that lacked lymph nodes. These results indicate that cutaneous exposure to food antigens can reprogram gut-homing effector T cells in LNs to express skin-homing receptors, eliciting skin lesions upon food allergen contact in orally sensitized AD patients.
Figures





References
-
- Niggemann B. Role of oral food challenges in the diagnostic work-up of food allergy in atopic eczema dermatitis syndrome. Allergy. 2004;59(suppl 78):32–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

