A dual role for the immune response in a mouse model of inflammation-associated lung cancer
- PMID: 21537082
- PMCID: PMC3104747
- DOI: 10.1172/JCI44796
A dual role for the immune response in a mouse model of inflammation-associated lung cancer
Abstract
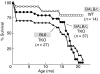
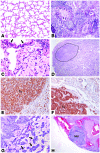
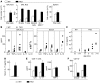
Lung cancer is the leading cause of cancer death worldwide. Both principal factors known to cause lung cancer, cigarette smoke and asbestos, induce pulmonary inflammation, and pulmonary inflammation has recently been implicated in several murine models of lung cancer. To further investigate the role of inflammation in the development of lung cancer, we generated mice with combined loss of IFN-γ and the β-common cytokines GM-CSF and IL-3. These immunodeficient mice develop chronic pulmonary inflammation and lung tumors at a high frequency. Examination of the relationship between these tumors and their inflammatory microenvironment revealed a dual role for the immune system in tumor development. The inflammatory cytokine IL-6 promoted optimal tumor growth, yet wild-type mice rejected transplanted tumors through the induction of adaptive immunity. These findings suggest a model whereby cytokine deficiency leads to oncogenic inflammation that combines with defective antitumor immunity to promote lung tumor formation, representing a unique system for studying the role of the immune system in lung tumor development.
Figures

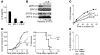
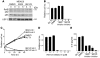
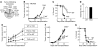
References
-
- Doll SR. Smoking and lung cancer. Am J Respir Crit Care Med. 2000;162(1):4–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

