Plasma membrane calcium ATPase proteins as novel regulators of signal transduction pathways
- PMID: 21537369
- PMCID: PMC3083965
- DOI: 10.4331/wjbc.v1.i6.201
Plasma membrane calcium ATPase proteins as novel regulators of signal transduction pathways
Abstract
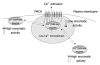
Emerging evidence suggests that plasma membrane calcium ATPases (PMCAs) play a key role as regulators of calcium-triggered signal transduction pathways via interaction with partner proteins. PMCAs regulate these pathways by targeting specific proteins to cellular sub-domains where the levels of intracellular free calcium are kept low by the calcium ejection properties of PMCAs. According to this model, PMCAs have been shown to interact functionally with the calcium-sensitive proteins neuronal nitric oxide synthase, calmodulin-dependent serine protein kinase, calcineurin and endothelial nitric oxidase synthase. Transgenic animals with altered expression of PMCAs are being used to evaluate the physiological significance of these interactions. To date, PMCA interactions with calcium-dependent partner proteins have been demonstrated to play a crucial role in the pathophysiology of the cardiovascular system via regulation of the nitric oxide and calcineurin/nuclear factor of activated T cells pathways. This new evidence suggests that PMCAs play a more sophisticated role than the mere ejection of calcium from the cells, by acting as modulators of signaling transduction pathways.
Keywords: Calcineurin; Nitric oxide; Nuclear factor of activated T cells; Plasma membrane calcium ATPase; Regulation; Signal transduction.
Figures
Similar articles
-
The interaction between endogenous calcineurin and the plasma membrane calcium-dependent ATPase is isoform specific in breast cancer cells.FEBS Lett. 2007 Aug 21;581(21):4115-9. doi: 10.1016/j.febslet.2007.07.054. Epub 2007 Jul 31. FEBS Lett. 2007. PMID: 17689535
-
Interaction of the plasma membrane Ca2+ pump 4b/CI with the Ca2+/calmodulin-dependent membrane-associated kinase CASK.J Biol Chem. 2003 Mar 14;278(11):9778-83. doi: 10.1074/jbc.M212507200. Epub 2003 Jan 2. J Biol Chem. 2003. PMID: 12511555
-
Regulation of GAP43/calmodulin complex formation via calcineurin-dependent mechanism in differentiated PC12 cells with altered PMCA isoforms composition.Mol Cell Biochem. 2015 Sep;407(1-2):251-62. doi: 10.1007/s11010-015-2473-4. Epub 2015 Jun 5. Mol Cell Biochem. 2015. PMID: 26045175 Free PMC article.
-
Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps.Physiol Rev. 2001 Jan;81(1):21-50. doi: 10.1152/physrev.2001.81.1.21. Physiol Rev. 2001. PMID: 11152753 Review.
-
Plasma membrane calcium ATPase and its relationship to nitric oxide signaling in the heart.Ann N Y Acad Sci. 2007 Mar;1099:247-53. doi: 10.1196/annals.1387.007. Ann N Y Acad Sci. 2007. PMID: 17446465 Review.
Cited by
-
Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: just What It Takes to Make a Blood Vessel.Int J Mol Sci. 2019 Aug 14;20(16):3962. doi: 10.3390/ijms20163962. Int J Mol Sci. 2019. PMID: 31416282 Free PMC article. Review.
-
The Impact of Vitamin D3 Supplementation on Mechanisms of Cell Calcium Signaling in Chronic Kidney Disease.Biomed Res Int. 2015;2015:807673. doi: 10.1155/2015/807673. Epub 2015 May 4. Biomed Res Int. 2015. PMID: 26064953 Free PMC article.
-
Plasma membrane calcium ATPases as novel candidates for therapeutic agent development.J Pharm Pharm Sci. 2013;16(2):190-206. doi: 10.18433/j3z011. J Pharm Pharm Sci. 2013. PMID: 23958189 Free PMC article. Review.
-
NFAT1 and NFAT3 cooperate with HDAC4 during regulation of alternative splicing of PMCA isoforms in PC12 cells.PLoS One. 2014 Jun 6;9(6):e99118. doi: 10.1371/journal.pone.0099118. eCollection 2014. PLoS One. 2014. PMID: 24905014 Free PMC article.
-
The plasma membrane calcium pump: new ways to look at an old enzyme.J Biol Chem. 2014 Apr 11;289(15):10261-10268. doi: 10.1074/jbc.O114.555565. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24570005 Free PMC article. Review.
References
-
- Carafoli E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 1994;8:993–1002. - PubMed
-
- Shull GE, Greeb J. Molecular cloning of two isoforms of the plasma membrane Ca2+-transporting ATPase from rat brain. Structural and functional domains exhibit similarity to Na+,K+- and other cation transport ATPases. J Biol Chem. 1988;263:8646–8657. - PubMed
-
- Greeb J, Shull GE. Molecular cloning of a third isoform of the calmodulin-sensitive plasma membrane Ca2+-transporting ATPase that is expressed predominantly in brain and skeletal muscle. J Biol Chem. 1989;264:18569–18576. - PubMed
-
- Verma AK, Filoteo AG, Stanford DR, Wieben ED, Penniston JT, Strehler EE, Fischer R, Heim R, Vogel G, Mathews S. Complete primary structure of a human plasma membrane Ca2+ pump. J Biol Chem. 1988;263:14152–14159. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

