Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells
- PMID: 21538216
- PMCID: PMC3160166
- DOI: 10.1208/s12248-011-9276-7
Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells
Abstract
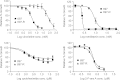
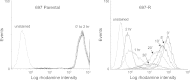
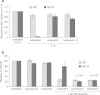
Protein synthesis is a powerful therapeutic target in leukemias and other cancers, but few pharmacologically viable agents are available that affect this process directly. The plant-derived agent silvestrol specifically inhibits translation initiation by interfering with eIF4A/mRNA assembly with eIF4F. Silvestrol has potent in vitro and in vivo activity in multiple cancer models including acute lymphoblastic leukemia (ALL) and is under pre-clinical development by the US National Cancer Institute, but no information is available about potential mechanisms of resistance. In a separate report, we showed that intraperitoneal silvestrol is approximately 100% bioavailable systemically, although oral doses were only 1% bioavailable despite an apparent lack of metabolism. To explore mechanisms of silvestrol resistance and the possible role of efflux transporters in silvestrol disposition, we characterized multi-drug resistance transporter expression and function in a silvestrol-resistant ALL cell line generated via culture of the 697 ALL cell line in gradually increasing silvestrol concentrations. This resistant cell line, 697-R, shows significant upregulation of ABCB1 mRNA and P-glycoprotein (Pgp) as well as cross-resistance to known Pgp substrates vincristine and romidepsin. Furthermore, 697-R cells readily efflux the fluorescent Pgp substrate rhodamine 123. This effect is prevented by Pgp inhibitors verapamil and cyclosporin A, as well as siRNA to ABCB1, with concomitant re-sensitization to silvestrol. Together, these data indicate that silvestrol is a substrate of Pgp, a potential obstacle that must be considered in the development of silvestrol for oral delivery or targeting to tumors protected by Pgp overexpression.
Figures



References
-
- Zimmer SG, DeBenedetti A, Graff JR. Translational control of malignancy: the mRNA cap-binding protein, eIF-4E, as a central regulator of tumor formation, growth, invasion and metastasis. Anticancer Res. 2000;20(3A):1343–1351. - PubMed
-
- Meric F, Hunt KK. Translation initiation in cancer: a novel target for therapy. Mol Cancer Ther. 2002;1(11):971–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

