NKG2A is a marker for acquisition of regulatory function by human CD8+ T cells activated with anti-CD3 antibody
- PMID: 21538351
- PMCID: PMC3517122
- DOI: 10.1002/eji.201041258
NKG2A is a marker for acquisition of regulatory function by human CD8+ T cells activated with anti-CD3 antibody
Abstract
Treatment with anti-CD3 mAb modulates immune responses that cause type 1 diabetes and other diseases. CD8+ Tregs can be induced in vitro and in vivo by mAb. However, 1/3 of patients do not respond to drug therapy and in an equal proportion, anti-CD3 mAb does not induce Tregs in vitro. The acquisition of CD8+ Treg activity is a function of the CD8+ cells and not the targets in the assay. To identify markers to differentiate responses of CD8+ Tregs, we analyzed genes differentially expressed in CD8+ T cells of non-responders compared with responders, and found that an inhibitory receptor NKG2A (CD159a) was highly expressed in cells from all non-responders tested. Application of a mAb agonistic to NKG2A during in vitro CD8+ Treg induction by anti-CD3 prevented induction of CD8+ Tregs. CD8+ T cells that are TNFR2+ but NKG2A- are the most potently induced Tregs. The level of NKG2A expression on resting CD8+ T cells inversely correlated with acquisition of regulatory function when activated. We suggest that the induction of human CD8+ Tregs by anti-CD3 mAb is controlled by a negative signaling through NKG2A, and that NKG2A may serve as a negative marker of human CD8+ Tregs.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
KH has received a grant from MacroGenics Inc to study patients treated with anti-CD3 mAb.
Figures
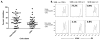
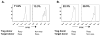



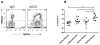

References
-
- Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A Single Course of Anti-CD3 Monoclonal Antibody hOKT3{gamma}1(Ala-Ala) Results in Improvement in C-Peptide Responses and Clinical Parameters for at Least 2 Years after Onset of Type 1 Diabetes. Diabetes. 2005;54:1763–1769. - PMC - PubMed
-
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

