Pairing phosphoinositides with calcium ions in endolysosomal dynamics: phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes
- PMID: 21538413
- PMCID: PMC3107950
- DOI: 10.1002/bies.201000152
Pairing phosphoinositides with calcium ions in endolysosomal dynamics: phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes
Abstract
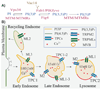
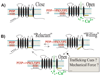
The direction and specificity of endolysosomal membrane trafficking is tightly regulated by various cytosolic and membrane-bound factors, including soluble NSF attachment protein receptors (SNAREs), Rab GTPases, and phosphoinositides. Another trafficking regulatory factor is juxta-organellar Ca(2+) , which is hypothesized to be released from the lumen of endolysosomes and to be present at higher concentrations near fusion/fission sites. The recent identification and characterization of several Ca(2+) channel proteins from endolysosomal membranes has provided a unique opportunity to examine the roles of Ca(2+) and Ca(2+) channels in the membrane trafficking of endolysosomes. SNAREs, Rab GTPases, and phosphoinositides have been reported to regulate plasma membrane ion channels, thereby suggesting that these trafficking regulators may also modulate endolysosomal dynamics by controlling Ca(2+) flux across endolysosomal membranes. In this paper, we discuss the roles of phosphoinositides, Ca(2+) , and potential interactions between endolysosomal Ca(2+) channels and phosphoinositides in endolysosomal dynamics.
Copyright © 2011 WILEY Periodicals, Inc.
Figures

Similar articles
-
Phosphoinositides as membrane organizers.Nat Rev Mol Cell Biol. 2022 Dec;23(12):797-816. doi: 10.1038/s41580-022-00490-x. Epub 2022 May 19. Nat Rev Mol Cell Biol. 2022. PMID: 35589852 Free PMC article. Review.
-
Two-pore channels at the intersection of endolysosomal membrane traffic.Biochem Soc Trans. 2015 Jun;43(3):434-41. doi: 10.1042/BST20140303. Biochem Soc Trans. 2015. PMID: 26009187 Free PMC article. Review.
-
Regulation of membrane trafficking by signalling on endosomal and lysosomal membranes.J Physiol. 2013 Sep 15;591(18):4389-401. doi: 10.1113/jphysiol.2013.258301. Epub 2013 Jul 22. J Physiol. 2013. PMID: 23878375 Free PMC article. Review.
-
Endolysosomal calcium release and cardiac physiology.Cell Calcium. 2022 Jun;104:102565. doi: 10.1016/j.ceca.2022.102565. Epub 2022 Feb 28. Cell Calcium. 2022. PMID: 35299075 Review.
-
Iron overload causes endolysosomal deficits modulated by NAADP-regulated 2-pore channels and RAB7A.Autophagy. 2016 Sep;12(9):1487-506. doi: 10.1080/15548627.2016.1190072. Epub 2016 Jul 6. Autophagy. 2016. PMID: 27383256 Free PMC article.
Cited by
-
Genetically encoded fluorescent probe to visualize intracellular phosphatidylinositol 3,5-bisphosphate localization and dynamics.Proc Natl Acad Sci U S A. 2013 Dec 24;110(52):21165-70. doi: 10.1073/pnas.1311864110. Epub 2013 Dec 9. Proc Natl Acad Sci U S A. 2013. PMID: 24324172 Free PMC article.
-
Two-pore channels (TPCs): Novel voltage-gated ion channels with pleiotropic functions.Channels (Austin). 2017 Jan 2;11(1):20-33. doi: 10.1080/19336950.2016.1213929. Epub 2016 Jul 20. Channels (Austin). 2017. PMID: 27440385 Free PMC article. Review.
-
Podocyte Lysosome Dysfunction in Chronic Glomerular Diseases.Int J Mol Sci. 2020 Feb 25;21(5):1559. doi: 10.3390/ijms21051559. Int J Mol Sci. 2020. PMID: 32106480 Free PMC article. Review.
-
Hormone-stimulated modulation of endocytic trafficking in osteoclasts.Front Endocrinol (Lausanne). 2012 Aug 22;3:103. doi: 10.3389/fendo.2012.00103. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22936925 Free PMC article.
-
PIPing on lysosome tubes.EMBO J. 2013 Feb 6;32(3):315-7. doi: 10.1038/emboj.2012.355. Epub 2013 Jan 11. EMBO J. 2013. PMID: 23314746 Free PMC article.
References
-
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. - PubMed
-
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. - PubMed
-
- de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–849. - PubMed
-
- Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8:355–368. - PubMed
-
- Bright NA, Gratian MJ, Luzio JP. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr Biol. 2005;15:360–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

