Protective role of cathepsin L in mouse skin carcinogenesis
- PMID: 21538579
- PMCID: PMC3155742
- DOI: 10.1002/mc.20792
Protective role of cathepsin L in mouse skin carcinogenesis
Abstract
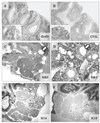
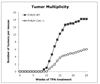
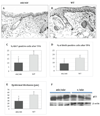
Lysosomal cysteine protease cathepsin L (CTSL) is believed to play a role in tumor progression and is considered a marker for clinically invasive tumors. Studies from our laboratory using the classical mouse skin carcinogenesis model, with 7,12-dimethyl-benz[a]anthracene (DMBA) for initiation and 12-O-tetradecanoylphorbol-13-acetate (TPA) for promotion, showed that expression of CTSL is increased in papillomas and squamous cell carcinomas (SCC). We also carried out carcinogenesis studies using Ctsl-deficient nackt (nkt) mutant mice on three different inbred backgrounds. Unexpectedly, the multiplicity of papillomas was significantly higher in Ctsl-deficient than in wild-type mice on two unrelated backgrounds. Topical applications of TPA or DMBA alone to the skin of nkt/nkt mice did not induce papillomas, and there was no increase in spontaneous tumors in nkt/nkt mice on any of the three inbred backgrounds. Reduced epidermal cell proliferation in Ctsl-deficient nkt/nkt mice after TPA treatment suggested that they are not more sensitive than wild-type mice to TPA promotion. We also showed that deficiency of CTSL delays terminal differentiation of keratinocytes, and we propose that decreased elimination of initiated cells is at least partially responsible for the increased papilloma formation in the nackt model.
Keywords: cysteine cathepsins; mouse models; proteases; skin cancer; two-stage carcinogenesis.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


References
-
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6(10):764–775. - PubMed
-
- Roth W, Deussing J, Botchkarev VA, et al. Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. Faseb J. 2000;14(13):2075–2086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

