Specificity of HCPTP variants toward EphA2 tyrosines by quantitative selected reaction monitoring
- PMID: 21538645
- PMCID: PMC3149191
- DOI: 10.1002/pro.646
Specificity of HCPTP variants toward EphA2 tyrosines by quantitative selected reaction monitoring
Abstract
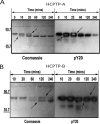
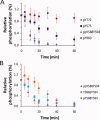
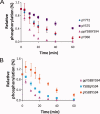
EphA2 receptor tyrosine kinase and the human cytoplasmic protein tyrosine phosphatase (HCPTP) are overexpressed in a number of epithelial cancers. Overexpressed EphA2 in these cancers shows a significant decrease in phosphotyrosine content which results in suppression of receptor signaling and endocytosis and an increase in metastatic potential. The decreased phosphotyrosine content of EphA2 has been associated with decreased contact with its ligand, ephrin A1 and dephosphorylation by HCPTP. Potential specificity of the two HCPTP variants for tyrosines on EphA2 has not been investigated. We have used a mass spectrometry assay to measure relative rates of dephosphorylation for the two HCPTP variants at phosphotyrosine sites associated with control of the EphA2 kinase activity or interaction with downstream targets. Our results suggest that although both variants dephosphorylate the EphA2 receptor, the rate and specificity of dephosphorylation for specific tyrosines are different for HCPTP-A and HCPTP-B. The SAM domain tyrosine Y960 which has been implicated in downstream PI3K signaling is dephosphorylated exclusively by HCPTP-B. The activation loop tyrosine (Y772) which directly controls kinase activity is dephosphorylated about six times faster by HCPTP-A. In contrast, the juxtamembrane tyrosines (Y575, Y588 and Y594) which are implicated in both control of kinase activity and downstream signaling are dephosphorylated by both variants with similar rates. This difference in preference for dephosphorylation sites on EphA2 not only illuminates the different roles of the two variants of the phosphatase in EphA2 signaling, but also explains why both HCPTP variants are highly conserved in most mammals.
Copyright © 2011 The Protein Society.
Figures


References
-
- Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. - PubMed
-
- Kinch MS, Carles-Kinch K. Overexpression and functional alterations of the EphA2 tyrosine kinase in cancer. Clin Exp Metastasis. 2003;20:59–68. - PubMed
-
- Zantek ND, Azimi M, Fedor-Chaiken M, Wang B, Brackenbury R, Kinch MS. E-Cadherin regulates the function of the EphA2 receptor tyrosine kinase 1. Cell Growth Differ. 1999;10:629–638. - PubMed
-
- Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

