Photosensitizer drug delivery via an optical fiber
- PMID: 21539365
- PMCID: PMC3329778
- DOI: 10.1021/ja200840p
Photosensitizer drug delivery via an optical fiber
Abstract
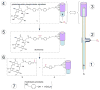
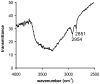
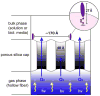
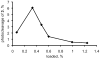
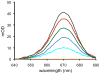
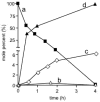
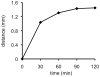
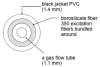
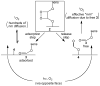
An optical fiber has been developed with a maneuverable mini-probe tip that sparges O(2) gas and photodetaches pheophorbide (sensitizer) molecules. Singlet oxygen is produced at the probe tip surface which reacts with an alkene spacer group releasing sensitizer upon fragmentation of a dioxetane intermediate. Optimal sensitizer photorelease occurred when the probe tip was loaded with 60 nmol sensitizer, where crowding of the pheophorbide molecules and self-quenching were kept to a minimum. The fiber optic tip delivered pheophorbide molecules and singlet oxygen to discrete locations. The 60 nmol sensitizer was delivered into petrolatum; however, sensitizer release was less efficient in toluene-d(8) (3.6 nmol) where most had remained adsorbed on the probe tip, even after the covalent alkene spacer bond had been broken. The results open the door to a new area of fiber optic-guided sensitizer delivery for the potential photodynamic therapy of hypoxic structures requiring cytotoxic control.
Figures


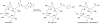
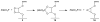
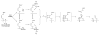
References
-
- Kessel D, Foster TH, editors. Symposium-In-Print: Photodynamic therapy. Photochem Photobiol. 2007;83:995–1282.
-
- Recommendations for occupational safety and health: Compendium of policy documents and statements. Cincinnati, OH: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Institute for Occupational Safety and Health; DHHS (NIOSH) Publication No. 92-100.
-
- Kammari L, Solomek T, Ngoy BP, Heger D, Klán P. J Am Chem Soc. 2010;132:11431–11433. - PubMed
- Xie Z, Hu X, Chen X, Sun J, Shi Q, Jing X. Biomacromolecules. 2008;9:376–380. - PubMed
- Kessler M, Glatthar R, Giese B, Bochet CG. Org Lett. 2003;5:1179–1181. - PubMed
- Singh AK, Khade PK. Bioconjugate Chem. 2002;13:1286–1291. - PubMed
- Hu J, Zhang J, Liu F, Kittredge K, Whitesell JK, Fox MA. J Am Chem Soc. 2001;123:1464–1470.
- Smet M, Liao LX, Dehaen W, McGrath DV. Org Lett. 2000;2:511–513. - PubMed
-
- Givens RS, Weber JFW, Conrad PG, Orosz G, Donahue SL, Thayer SA. J Am Chem Soc. 2000;122:2687–2697.
- Givens R. In: CRC Handbook of Organic Photochemistry and Photobiology. 2. Horspool WM, Lenci F, editors. CRC Press; Boca Raton, FL: 2003. pp. 1–46.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

