C5b-9-activated, K(v)1.3 channels mediate oligodendrocyte cell cycle activation and dedifferentiation
- PMID: 21540025
- PMCID: PMC3139709
- DOI: 10.1016/j.yexmp.2011.04.006
C5b-9-activated, K(v)1.3 channels mediate oligodendrocyte cell cycle activation and dedifferentiation
Abstract
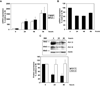
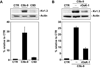
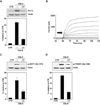
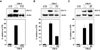
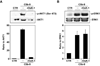
Voltage-gated potassium (K(v)) channels play an important role in the regulation of growth factor-induced cell proliferation. We have previously shown that cell cycle activation is induced in oligodendrocytes (OLGs) by complement C5b-9, but the role of K(v) channels in these cells had not been investigated. Differentiated OLGs were found to express K(v)1.4 channels, but little K(v)1.3. Exposure of OLGs to C5b-9 modulated K(v)1.3 functional channels and increased protein expression, whereas C5b6 had no effect. Pretreatment with the recombinant scorpion toxin rOsK-1, a highly selective K(v)1.3 inhibitor, blocked the expression of K(v)1.3 induced by C5b-9. rOsK-1 inhibited Akt phosphorylation and activation by C5b-9 but had no effect on ERK1 activation. These data strongly suggest a role for K(v)1.3 in controlling the Akt activation induced by C5b-9. Since Akt plays a major role in C5b-9-induced cell cycle activation, we also investigated the effect of inhibiting K(v)1.3 channels on DNA synthesis. rOsK-1 significantly inhibited the DNA synthesis induced by C5b-9 in OLG, indicating that K(v)1.3 plays an important role in the C5b-9-induced cell cycle. In addition, C5b-9-mediated myelin basic protein and proteolipid protein mRNA decay was completely abrogated by inhibition of K(v)1.3 expression. In the brains of multiple sclerosis patients, C5b-9 co-localized with NG2(+) OLG progenitor cells that expressed K(v)1.3 channels. Taken together, these data suggest that K(v)1.3 channels play an important role in controlling C5b-9-induced cell cycle activation and OLG dedifferentiation, both in vitro and in vivo.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Bacia A, et al. K+ channel blockade impairs remyelination in the cuprizone model. Glia. 2004;48:156–165. - PubMed
-
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–468. - PubMed
-
- Bever CT, Judge SI. Sustained-release fampridine for multiple sclerosis. Expert Opin Investig Drugs. 2009;18:1013–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

