Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity
- PMID: 21540183
- PMCID: PMC3121368
- DOI: 10.1074/jbc.M111.228874
Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity
Abstract
SIRT1 is involved in the pathogenesis of obesity, diabetes, and aging. However, it is not clear how SIRT1 activity is regulated by intracellular kinases in cells. In this study, we investigated SIRT1 phosphorylation and protein degradation in response to JNK1 activation in obese mice. Mouse SIRT1 is phosphorylated by JNK1 at Ser-46 (Ser-47 in human SIRT1), which is one of the four potential residues targeted by JNK1. The phosphorylation induces a brief activation of SIRT1 function and degradation of SIRT1 thereafter by the proteasome. Ubiquitination occurs in SIRT1 protein after the phosphorylation. Mutation of Ser-46 to alanine prevents the phosphorylation, ubiquitination, and degradation. In vivo, SIRT1 undergoes an extensive degradation in hepatocytes in obesity as a consequence of persistent activation of JNK1. The degradation leads to inhibition of SIRT1 function, which contributes to development of hepatic steatosis. The degradation disappears in obesity when JNK1 is inactivated in mice. JNK2 exhibits an opposite activity in the regulation of SIRT1 degradation. The JNK1-SIRT1 pathway provides a new molecular mechanism for the pathogenesis of hepatic steatosis in obesity.
Figures




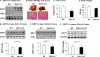

References
-
- Blander G., Guarente L. (2004) Annu. Rev. Biochem. 73, 417–435 - PubMed
-
- Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Nature 403, 795–800 - PubMed
-
- Lin S. J., Defossez P. A., Guarente L. (2000) Science 289, 2126–2128 - PubMed
-
- Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nature 434, 113–118 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

