Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study
- PMID: 21540198
- PMCID: PMC3103669
- DOI: 10.1136/ard.2010.144063
Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study
Abstract
Objective: This study assessed the efficacy and safety of canakinumab, a fully human anti-interleukin 1β monoclonal antibody, for prophylaxis against acute gouty arthritis flares in patients initiating urate-lowering treatment.
Methods: In this double-blind, double-dummy, dose-ranging study, 432 patients with gouty arthritis initiating allopurinol treatment were randomised 1:1:1:1:1:1:2 to receive: a single dose of canakinumab, 25, 50, 100, 200, or 300 mg subcutaneously; 4×4-weekly doses of canakinumab (50+50+25+25 mg subcutaneously); or daily colchicine 0.5 mg orally for 16 weeks. Patients recorded details of flares in diaries. The study aimed to determine the canakinumab dose having equivalent efficacy to colchicine 0.5 mg at 16 weeks.
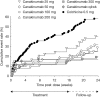
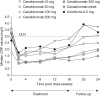
Results: A dose-response for canakinumab was not apparent with any of the four predefined dose-response models. The estimated canakinumab dose with equivalent efficacy to colchicine was below the range of doses tested. At 16 weeks, there was a 62% to 72% reduction in the mean number of flares per patient for canakinumab doses ≥50 mg versus colchicine based on a negative binomial model (rate ratio: 0.28-0.38, p≤0.0083), and the percentage of patients experiencing ≥1 flare was significantly lower for all canakinumab doses (15% to 27%) versus colchicine (44%, p<0.05). There was a 64% to 72% reduction in the risk of experiencing ≥1 flare for canakinumab doses ≥50 mg versus colchicine at 16 weeks (hazard ratio (HR): 0.28-0.36, p≤0.05). The incidence of adverse events was similar across treatment groups.
Conclusions: Single canakinumab doses ≥50 mg or four 4-weekly doses provided superior prophylaxis against flares compared with daily colchicine 0.5 mg.
Conflict of interest statement
Figures

References
-
- Kramer HM, Curhan G. The association between gout and nephrolithiasis: the National Health and Nutrition Examination Survey III, 1988-1994. Am J Kidney Dis 2002;40:37–42 - PubMed
-
- Schlesinger N. Diagnosis of gout. Minerva Med 2007;98:759–67 - PubMed
-
- Schlesinger N. Diagnosis of gout: clinical, laboratory, and radiologic findings. Am J Manag Care 2005;11(15 Suppl):S443–50; quiz S465–8 - PubMed
-
- Keenan RT, O'Brien WR, Lee KH, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med 2011;124:155–63 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

