Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes
- PMID: 21540339
- PMCID: PMC3167111
- DOI: 10.1113/jphysiol.2011.207829
Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes
Abstract
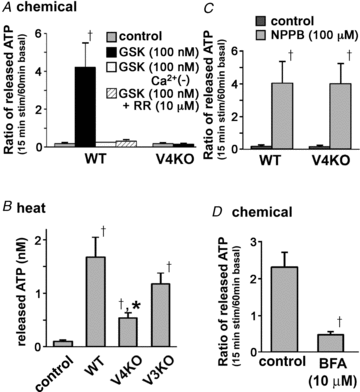
Gastro-oesophageal reflux disease (GERD) is a multi-factorial disease that may involve oesophageal hypersensitivity to mechanical or heat stimulus as well as acids. Intraganglionic laminar endings (IGLEs) are the most prominent terminal structures of oesophageal vagal mechanosensitive afferents and may modulate mechanotransduction via purinergic receptors. Transient receptor potential channel vanilloid 4 (TRPV4) can detect various stimuli such as warm temperature, stretch and some chemicals, including 4α-phorbol 12,13-didecanoate (4α-PDD) and GSK1016790A. TRPV4 is expressed in many tissues, including renal epithelium, skin keratinocytes and urinary bladder epithelium, but its expression and function in the oesophagus is poorly understood. Here, we show anatomical and functional TRPV4 expression in mouse oesophagus and its involvement in ATP release. TRPV4 mRNA and protein were detected in oesophageal keratinocytes. Several known TRPV4 activators (chemicals, heat and stretch stimulus) increased cytosolic Ca2+ concentrations in cultured WT keratinocytes but not in TRPV4 knockout (KO) cells. Moreover, the TRPV4 agonist GSK1016790A and heat stimulus evoked TRPV4-like current responses in isolated WT keratinocytes, but not in TRPV4KO cells. GSK1016790A and heat stimulus also significantly increased ATP release from WT oesophageal keratinocytes compared to TRPV4KO cells. The vesicle-trafficking inhibitor brefeldin A (BFA) inhibited the ATP release. This ATP release could be mediated by the newly identified vesicle ATP transporter, VNUT, which is expressed by oesophageal keratinocytes at the mRNA and protein levels. In conclusion, in response to heat, chemical and possibly mechanical stimuli, TRPV4 contributes to ATP release in the oesophagus. Thus, TRPV4 could be involved in oesophageal mechano- and heat hypersensitivity.
Figures



Comment in
-
TRiPping down the oesophagus.J Physiol. 2011 Jul 15;589(Pt 14):3415-6. doi: 10.1113/jphysiol.2011.212977. J Physiol. 2011. PMID: 21764758 Free PMC article. No abstract available.
References
-
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. - PubMed
-
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. - PubMed
-
- Burnstock G, Cocks T, Kasakov L, Wong HK. Direct evidence for ATP release from non-adrenergic, non-cholinergic (‘purinergic’) nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978;49:145–149. - PubMed
-
- Dent J. Microscopic esophageal mucosal injury in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2007;5:4–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

