Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis
- PMID: 21540554
- PMCID: PMC3083792
- DOI: 10.1172/JCI43615
Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis
Abstract
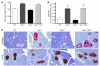
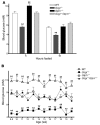
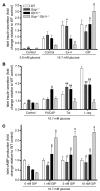
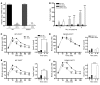
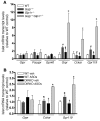
Disordered glucagon secretion contributes to the symptoms of diabetes, and reduced glucagon action is known to improve glucose homeostasis. In mice, genetic deletion of the glucagon receptor (Gcgr) results in increased levels of the insulinotropic hormone glucagon-like peptide 1 (GLP-1), which may contribute to the alterations in glucose homeostasis observed in Gcgr-/- mice. Here, we assessed the contribution of GLP-1 receptor (GLP-1R) signaling to the phenotype of Gcgr-/- mice by generating Gcgr-/-Glp1r-/- mice. Although insulin sensitivity was similar in all genotypes, fasting glucose was increased in Gcgr-/-Glp1r-/- mice. Elimination of the Glp1r normalized gastric emptying and impaired intraperitoneal glucose tolerance in Gcgr-/- mice. Unexpectedly, deletion of Glp1r in Gcgr-/- mice did not alter the improved oral glucose tolerance and increased insulin secretion characteristic of that genotype. Although Gcgr-/-Glp1r-/- islets exhibited increased sensitivity to the incretin glucose-dependent insulinotropic polypeptide (GIP), mice lacking both Glp1r and the GIP receptor (Gipr) maintained preservation of the enteroinsular axis following reduction of Gcgr signaling. Moreover, Gcgr-/-Glp1r-/- islets expressed increased levels of the cholecystokinin A receptor (Cckar) and G protein-coupled receptor 119 (Gpr119) mRNA transcripts, and Gcgr-/-Glp1r-/- mice exhibited increased sensitivity to exogenous CCK and the GPR119 agonist AR231453. Our data reveal extensive functional plasticity in the enteroinsular axis via induction of compensatory mechanisms that control nutrient-dependent regulation of insulin secretion.
Figures

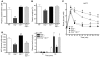

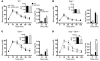


References
-
- Jaspan JB, Rubinstein AH. Circulating glucagon: plasma profiles and metabolism in health and disease. Diabetes. 1977;26(9):887–902. - PubMed
-
- Sherwin RS, Fisher M, Hendler R, Felig P. Hyperglucagonemia and blood glucose regulation in normal, obese and diabetic subjects. N Engl J Med. 1976;294(9):455–461. - PubMed
-
- Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28(8):574–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

