HER2 evaluation and its impact on breast cancer treatment decisions
- PMID: 21540562
- PMCID: PMC3225235
- DOI: 10.1159/000325746
HER2 evaluation and its impact on breast cancer treatment decisions
Abstract
Background: Eighteen to twenty percent of breast cancer tumors show abnormal amplification of the Human Epidermal growth factor Receptor 2 (HER2) gene and increased expression of the associated protein. HER2 amplification is associated with rapid tumor proliferation and shorter disease-free and overall survival. Because women with HER2 amplification are more likely to benefit from treatment with the drug trastuzumab, testing for HER2 is recommended to guide therapy. However, little is known about use of HER2 testing in real-world settings. This study examined uptake, use, appropriateness of HER2 testing, and the relationship between HER2 test results and treatment decisions.
Methods: We assessed electronic data from 3,634 patients with invasive breast cancer diagnosed from 1998 to 2007 in a large integrated health system. We collected data on patient and tumor characteristics, HER2 testing status, test results, and trastuzumab treatment.
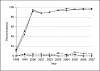
Results: From 1998 to 2000, the percent of patients who underwent HER2 evaluation increased from 12 to 94%; <3% of women with ductal carcinoma in situ, for whom HER2 testing is not recommended, were tested. Trastuzumab use increased 5-fold after 2004, when guidelines expanded to include recommending adjuvant treatment for early-stage breast cancer in addition to metastatic treatment. Ninety-five percent of women receiving trastuzumab had a positive HER2 result. After 2004, 55% of women with invasive breast cancer and overexpression of HER2 received trastuzumab treatment; this ranged from 44% of women with localized breast cancer to 80% of women with distant metastatic disease.
Conclusions: These findings illustrate appropriate and effective implementation of a HER2 testing strategy in a managed care setting.
Copyright © 2011 S. Karger AG, Basel.
Figures
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- American Cancer Society: Cancer facts & figures 2009. Atlanta, 2009. http://www.scribd.com/doc/21686287/Cancer-Facts-and-Figures-Report-2009
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

