Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts
- PMID: 21541185
- PMCID: PMC3085479
- DOI: 10.1155/2011/506171
Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts
Abstract
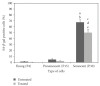
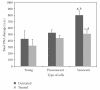
This study determined the molecular mechanisms of tocotrienol-rich fraction (TRF) in preventing cellular senescence of human diploid fibroblasts (HDFs). Primary culture of HDFs at various passages were incubated with 0.5 mg/mL TRF for 24 h. Telomere shortening with decreased telomerase activity was observed in senescent HDFs while the levels of damaged DNA and number of cells in G(0)/G(1) phase were increased and S phase cells were decreased. Incubation with TRF reversed the morphology of senescent HDFs to resemble that of young cells with decreased activity of SA-β-gal, damaged DNA, and cells in G(0)/G(1) phase while cells in the S phase were increased. Elongated telomere length and restoration of telomerase activity were observed in TRF-treated senescent HDFs. These findings confirmed the ability of tocotrienol-rich fraction in preventing HDFs cellular ageing by restoring telomere length and telomerase activity, reducing damaged DNA, and reversing cell cycle arrest associated with senescence.
Figures





References
-
- Hayflick L, Moorehead PS. The serial cultivation of human diploid cell strains. Experimental Cell Research. 1961;25(3):585–621. - PubMed
-
- Yeo EJ, Hwang YC, Kang CM, et al. Senescence-like changes induced by hydroxyurea in human diploid fibroblasts. Experimental Gerontology. 2000;35(5):553–571. - PubMed
-
- Campisi J. Cancer, aging and cellular senescence. In Vivo. 2000;14(1):183–188. - PubMed
-
- Duan J, Duan J, Zhang Z, Tong T. Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. International Journal of Biochemistry and Cell Biology. 2005;37(7):1407–1420. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Current Opinion in Cell Biology. 2003;15(2):247–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

