Amyloid beta resistance in nerve cell lines is mediated by the Warburg effect
- PMID: 21541279
- PMCID: PMC3082554
- DOI: 10.1371/journal.pone.0019191
Amyloid beta resistance in nerve cell lines is mediated by the Warburg effect
Abstract
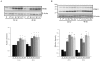
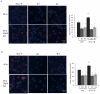
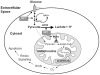
Amyloid beta (Aβ) peptide accumulation in the brains of patients with Alzheimer's disease (AD) is closely associated with increased nerve cell death. However, many cells survive and it is important to understand the mechanisms involved in this survival response. Recent studies have shown that an anti-apoptotic mechanism in cancer cells is mediated by aerobic glycolysis, also known as the Warburg effect. One of the major regulators of aerobic glycolysis is pyruvate dehydrogenase kinase (PDK), an enzyme which represses mitochondrial respiration and forces the cell to rely heavily on glycolysis, even in the presence of oxygen. Recent neuroimaging studies have shown that the spatial distribution of aerobic glycolysis in the brains of AD patients strongly correlates with Aβ deposition. Interestingly, clonal nerve cell lines selected for resistance to Aβ exhibit increased glycolysis as a result of activation of the transcription factor hypoxia inducible factor 1. Here we show that Aβ resistant nerve cell lines upregulate Warburg effect enzymes in a manner reminiscent of cancer cells. In particular, Aβ resistant nerve cell lines showed elevated PDK1 expression in addition to an increase in lactate dehydrogenase A (LDHA) activity and lactate production when compared to control cells. In addition, mitochondrial derived reactive oxygen species (ROS) were markedly diminished in resistant but not sensitive cells. Chemically or genetically inhibiting LDHA or PDK1 re-sensitized resistant cells to Aβ toxicity. These findings suggest that the Warburg effect may contribute to apoptotic-resistance mechanisms in the surviving neurons of the AD brain. Loss of the adaptive advantage afforded by aerobic glycolysis may exacerbate the pathophysiological processes associated with AD.
Conflict of interest statement
Figures



Similar articles
-
Overexpression of pyruvate dehydrogenase kinase 1 and lactate dehydrogenase A in nerve cells confers resistance to amyloid β and other toxins by decreasing mitochondrial respiration and reactive oxygen species production.J Biol Chem. 2012 Oct 26;287(44):37245-58. doi: 10.1074/jbc.M112.366195. Epub 2012 Sep 4. J Biol Chem. 2012. PMID: 22948140 Free PMC article.
-
A disease with a sweet tooth: exploring the Warburg effect in Alzheimer's disease.Biogerontology. 2017 Jun;18(3):301-319. doi: 10.1007/s10522-017-9692-x. Epub 2017 Mar 17. Biogerontology. 2017. PMID: 28314935 Review.
-
beta-Amyloid neurotoxicity is exacerbated during glycolysis inhibition and mitochondrial impairment in the rat hippocampus in vivo and in isolated nerve terminals: implications for Alzheimer's disease.Exp Neurol. 2002 Jul;176(1):163-74. doi: 10.1006/exnr.2002.7912. Exp Neurol. 2002. PMID: 12093093
-
PDK1 inhibition is a novel therapeutic target in multiple myeloma.Br J Cancer. 2013 Jan 15;108(1):170-8. doi: 10.1038/bjc.2012.527. Epub 2012 Nov 29. Br J Cancer. 2013. PMID: 23321518 Free PMC article.
-
Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1.Biochem J. 2007 Jul 1;405(1):1-9. doi: 10.1042/BJ20070389. Biochem J. 2007. PMID: 17555402 Review.
Cited by
-
Therapeutic Potential of Targeting Mitochondria for Alzheimer's Disease Treatment.J Clin Med. 2022 Nov 14;11(22):6742. doi: 10.3390/jcm11226742. J Clin Med. 2022. PMID: 36431219 Free PMC article. Review.
-
Effects of Combining Biofactors on Bioenergetic Parameters, Aβ Levels and Survival in Alzheimer Model Organisms.Int J Mol Sci. 2022 Aug 4;23(15):8670. doi: 10.3390/ijms23158670. Int J Mol Sci. 2022. PMID: 35955803 Free PMC article.
-
Brain aerobic glycolysis functions and Alzheimer's disease.Clin Transl Imaging. 2015 Feb 1;3(1):27-37. doi: 10.1007/s40336-014-0094-7. Epub 2014 Dec 10. Clin Transl Imaging. 2015. PMID: 26855936 Free PMC article.
-
Evaluation of Metabolic and Synaptic Dysfunction Hypotheses of Alzheimer's Disease (AD): A Meta-Analysis of CSF Markers.Curr Alzheimer Res. 2018;15(2):164-181. doi: 10.2174/1567205014666170921122458. Curr Alzheimer Res. 2018. PMID: 28933272 Free PMC article. Review.
-
Fueling the brain - the role of apolipoprotein E in brain energy metabolism and its implications for Alzheimer's disease.Transl Psychiatry. 2025 Aug 25;15(1):316. doi: 10.1038/s41398-025-03550-w. Transl Psychiatry. 2025. PMID: 40855008 Free PMC article. Review.
References
-
- Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. - PubMed
-
- Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–159. - PubMed
-
- Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998;8:447–453. - PubMed
-
- Behl C. Amyloid beta-protein toxicity and oxidative stress in Alzheimer's disease. Cell Tissue Res. 1997;290:471–480. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

