Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons
- PMID: 21541286
- PMCID: PMC3082561
- DOI: 10.1371/journal.pone.0019285
Differential roles for STIM1 and STIM2 in store-operated calcium entry in rat neurons
Abstract
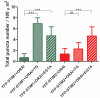
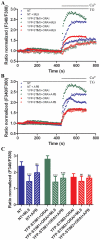
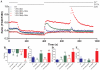
The interaction between Ca(2+) sensors STIM1 and STIM2 and Ca(2+) channel-forming protein ORAI1 is a crucial element of store-operated calcium entry (SOCE) in non-excitable cells. However, the molecular mechanism of SOCE in neurons remains unclear. We addressed this issue by establishing the presence and function of STIM proteins. Real-time polymerase chain reaction from cortical neurons showed that these cells contain significant amounts of Stim1 and Stim2 mRNA. Thapsigargin (TG) treatment increased the amount of both endogenous STIM proteins in neuronal membrane fractions. The number of YFP-STIM1/ORAI1 and YFP-STIM2/ORAI1 complexes was also enhanced by such treatment. The differences observed in the number of STIM1 and STIM2 complexes under SOCE conditions and the differential sensitivity to SOCE inhibitors suggest their distinct roles. Endoplasmic reticulum (ER) store depletion by TG enhanced intracellular Ca(2+) levels in loaded with Fura-2 neurons transfected with YFP-STIM1 and ORAI1, but not with YFP-STIM2 and ORAI1, which correlated well with the number of complexes formed. Moreover, the SOCE inhibitors ML-9 and 2-APB reduced Ca(2+) influx in neurons expressing YFP-STIM1/ORAI1 but produced no effect in cells transfected with YFP-STIM2/ORAI1. Moreover, in neurons transfected with YFP-STIM2/ORAI1, the increase in constitutive calcium entry was greater than with YFP-STIM1/ORAI1. Our data indicate that both STIM proteins are involved in calcium homeostasis in neurons. STIM1 mainly activates SOCE, whereas STIM2 regulates resting Ca(2+) levels in the ER and Ca(2+) leakage with the additional involvement of STIM1.
Conflict of interest statement
Figures



References
-
- Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
-
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci. 2001;24:602–608. - PubMed
-
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. - PubMed
-
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

