α-synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson's disease patients
- PMID: 21541339
- PMCID: PMC3081826
- DOI: 10.1371/journal.pone.0018513
α-synuclein reactive antibodies as diagnostic biomarkers in blood sera of Parkinson's disease patients
Abstract
Background: Auto-antibodies with specificity to self-antigens have been implicated in a wide variety of neurological diseases, including Parkinson's (PD) and Alzheimer's diseases, being sensitive indicators of neurodegeneration and focus for disease prevention. Of particular interest are the studies focused on the auto-immune responses to amyloidogenic proteins associated with diseases and their applications in therapeutic treatments such as vaccination with amyloid antigens and antibodies in PD, Alzheimer's disease and potentially other neurodegeneration ailments.
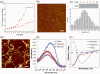
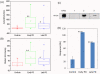
Methodology/principal findings: Generated auto-antibodies towards the major amyloidogenic protein involved in PD Lewy bodies--α-synuclein and its amyloid oligomers and fibrils were measured in the blood sera of early and late PD patients and controls by using ELISA, Western blot and Biacore surface plasmon resonance. We found significantly higher antibody levels towards monomeric α-synuclein in the blood sera of PD patients compared to controls, though the responses decreased with PD progression (P<0.0001). This indicates potential protective role of autoimmunity in maintaining the body homeostasis and clearing protein species whose disbalance may lead to amyloid assembly. There were no noticeable immune responses towards amyloid oligomers, but substantially increased levels of IgGs towards α-synuclein amyloid fibrils both in PD patients and controls, which subsided with the disease progression (P<0.0001). Pooled IgGs from PD patients and controls interacted also with the amyloid fibrils of Aβ (1-40) and hen lysozyme, however the latter were recognized with lower affinity. This suggests that IgGs bind to the generic amyloid conformational epitope, displaying higher specificity towards human amyloid species associated with neurodegeneration.
Conclusions/significance: Our findings may suggest the protective role of autoimmunity in PD and therefore immune reactions towards PD major amyloid protein--α-synuclein can be of value in the development of treatment and diagnostic strategies, especially during the early disease stages.
Conflict of interest statement
Figures





References
-
- Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909. - PubMed
-
- Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. - PubMed
-
- Martin JB. Molecular basis of the neurodegenerative disorders. N Engl J Med. 1999;340:1970–1980. - PubMed
-
- Selkoe DJ. Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–631. - PubMed
-
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

