Full-length L1CAM and not its Δ2Δ27 splice variant promotes metastasis through induction of gelatinase expression
- PMID: 21541352
- PMCID: PMC3081839
- DOI: 10.1371/journal.pone.0018989
Full-length L1CAM and not its Δ2Δ27 splice variant promotes metastasis through induction of gelatinase expression
Abstract
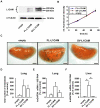
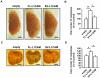
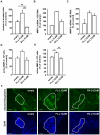
Tumour-specific splicing is known to contribute to cancer progression. In the case of the L1 cell adhesion molecule (L1CAM), which is expressed in many human tumours and often linked to bad prognosis, alternative splicing results in a full-length form (FL-L1CAM) and a splice variant lacking exons 2 and 27 (SV-L1CAM). It has not been elucidated so far whether SV-L1CAM, classically considered as tumour-associated, or whether FL-L1CAM is the metastasis-promoting isoform. Here, we show that both variants were expressed in human ovarian carcinoma and that exposure of tumour cells to pro-metastatic factors led to an exclusive increase of FL-L1CAM expression. Selective overexpression of one isoform in different tumour cells revealed that only FL-L1CAM promoted experimental lung and/or liver metastasis in mice. In addition, metastasis formation upon up-regulation of FL-L1CAM correlated with increased invasive potential and elevated Matrix metalloproteinase (MMP)-2 and -9 expression and activity in vitro as well as enhanced gelatinolytic activity in vivo. In conclusion, we identified FL-L1CAM as the metastasis-promoting isoform, thereby exemplifying that high expression of a so-called tumour-associated variant, here SV-L1CAM, is not per se equivalent to a decisive role of this isoform in tumour progression.
Conflict of interest statement
Figures

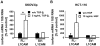
References
-
- Moos M, Tacke R, Scherer H, Teplow D, Fruh K, et al. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701–703. - PubMed
-
- Reid RA, Hemperly JJ. Variants of human L1 cell adhesion molecule arise through alternate splicing of RNA. J Mol Neurosci. 1992;3:127–135. - PubMed
-
- Breitbart RE, Andreadis A, Nadal-Ginard B. Alternative splicing: a ubiquitous mechanism for the generation of multiple protein isoforms from single genes. Annu Rev Biochem. 1987;56:467–495. - PubMed
-
- Pajares MJ, Ezponda T, Catena R, Calvo A, Pio R, et al. Alternative splicing: an emerging topic in molecular and clinical oncology. Lancet Oncol. 2007;8:349–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

