Sequential morphological characteristics of murine fetal liver hematopoietic microenvironment in Swiss Webster mice
- PMID: 21541657
- PMCID: PMC3102842
- DOI: 10.1007/s00441-011-1170-1
Sequential morphological characteristics of murine fetal liver hematopoietic microenvironment in Swiss Webster mice
Abstract
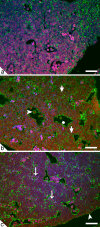
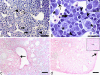
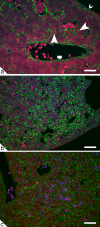
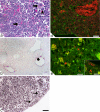
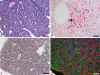
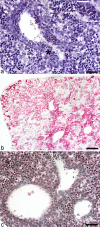
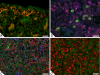
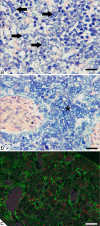
Embryonic hematopoiesis occurs via dynamic development with cells migrating into various organs. Fetal liver is the main hematopoietic organ responsible for hematopoietic cell expansion during embryologic development. We describe the morphological sequential characteristics of murine fetal liver niches that favor the settlement and migration of hematopoietic cells from 12 days post-coitum (dpc) to 0 day post-partum. Liver sections were stained with hematoxylin and eosin, Lennert's Giemsa, Sirius Red pH 10.2, Gomori's Reticulin, and Periodic Acid Schiff/Alcian Blue pH 1.0 and pH 2.5 and were analyzed by bright-field microscopy. Indirect imunohistochemistry for fibronectin, matrix metalloproteinase-1 (MMP-1), and MMP-9 and histochemistry for naphthol AS-D chloroacetate esterase (NCAE) were analyzed by confocal microscopy. The results showed that fibronectin was related to the promotion of hepatocyte and trabecular differentiation; reticular fibers did not appear to participate in fetal hematopoiesis but contributed to the physical support of the liver after 18 dpc. During the immature phase, hepatocytes acted as the fundamental stroma for the erythroid lineage. The appearance of myeloid cells in the liver was related to perivascular and subcapsular collagen, and NCAE preceded MMP-1 expression in neutrophils, an occurrence that appeared to contribute to their liver evasion. Thus, the murine fetal liver during ontogenesis shows two different phases: one immature and mainly endodermic (<14 dpc) and the other more developed (endodermic-mesenchymal; >15 dpc) with the maturation of hepatocytes, a better definition of trabecular pattern, and an increase in the connective tissue in the capsule, portal spaces, and liver parenchyma. The decrease of hepatic hematopoiesis (migration) coincides with hepatic maturation.
Figures




References
-
- Andrade ZA. Esquistosomiasis hepática: aspectos morfológicos. In: Popper H, Schaffner F, editors. Progresos en patología hepática, vol II. Barcelona: Editorial Científico Médica; 1967. pp. 249–264.
-
- Arias MA, Stewart A. Molecular principles of animal development. Oxford: Oxford University Press; 2002.
-
- Bogomoletz W. Advantages of the Sirius red staining method for amyloid and eosinophils. Arch Anat Cytol Pathol. 1980;28:252–253. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

