MALDI tissue imaging: from biomarker discovery to clinical applications
- PMID: 21541816
- PMCID: PMC6037172
- DOI: 10.1007/s00216-011-5003-6
MALDI tissue imaging: from biomarker discovery to clinical applications
Abstract
Matrix-assisted laser desorption ionization (MALDI) imaging mass spectrometry (IMS) is a powerful tool for the generation of multidimensional spatial expression maps of biomolecules directly from a tissue section. From a clinical proteomics perspective, this method correlates molecular detail to histopathological changes found in patient-derived tissues, enhancing the ability to identify candidates for disease biomarkers. The unbiased analysis and spatial mapping of a variety of molecules directly from clinical tissue sections can be achieved through this method. Conversely, targeted IMS, by the incorporation of laser-reactive molecular tags onto antibodies, aptamers, and other affinity molecules, enables analysis of specific molecules or a class of molecules. In addition to exploring tissue during biomarker discovery, the integration of MALDI-IMS methods into existing clinical pathology laboratory practices could prove beneficial to diagnostics. Querying tissue for the expression of specific biomarkers in a biopsy is a critical component in clinical decision-making and such markers are a major goal of translational research. An important challenge in cancer diagnostics will be to assay multiple parameters in a single slide when tissue quantities are limited. The development of multiplexed assays that maximize the yield of information from a small biopsy will help meet a critical challenge to current biomarker research. This review focuses on the use of MALDI-IMS in biomarker discovery and its potential as a clinical diagnostic tool with specific reference to our application of this technology to prostate cancer.
Figures


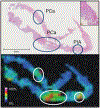



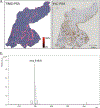
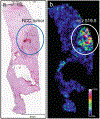
References
-
- Hillenkamp F et al. (1991) Anal Chem 63(24):1193A–1203A - PubMed
-
- Vestal ML (2009) J Mass Spectrom 44(3):303–317 - PubMed
-
- Cazares LH et al. (2009) Clin Cancer Res 15(17):5541–5551 - PubMed
-
- Agar NY et al. (2010) Methods Mol Biol 656:415–431 - PubMed
-
- Caldwell RL, Caprioli RM (2005) Mol Cell Proteomics 4(4):394–401 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

