Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort
- PMID: 21542002
- PMCID: PMC3179299
- DOI: 10.1002/jbmr.288
Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort
Abstract
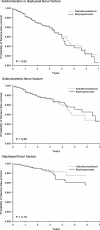
Bisphosphonates are the primary therapy for postmenopausal and glucocorticoid-induced osteoporosis. Case series suggest a potential link between prolonged use of bisphosphonates and low-energy fracture of subtrochanteric or diaphyseal femur as a consequence of oversuppression of bone resorption. Using health care utilization data, we conducted a propensity score-matched cohort study to examine the incidence rates (IRs) and risk of subtrochanteric or diaphyseal femur fractures among oral bisphosphonate users compared with raloxifene or calcitonin users. A Cox proportional hazards model evaluated the risk of these fractures associated with duration of osteoporosis treatment. A total of 104 subtrochanteric or diaphyseal femur fractures were observed among 33,815 patients. The estimated IR of subtrochanteric or diaphyseal femur fractures per 1000 person-years was 1.46 [95% confidence interval (CI) 1.11-1.88] among the bisphosphonate users and 1.43 (95% CI 1.06-1.89) among raloxifene/calcitonin users. No significant association between bisphosphonate use and subtrochanteric or diaphyseal femur fractures was found [hazard ratio (HR) = 1.03, 95% CI 0.70-1.52] compared with raloxifene/calcitonin. Even with this large study size, we had little precision in estimating the risk of subtrochanteric or diaphyseal femur fractures in patients treated with bisphosphonates for longer than 5 years (HR = 2.02, 95% CI 0.41-10.00). The occurrence of subtrochanteric or diaphyseal femur fracture was rare. There was no evidence of an increased risk of subtrochanteric or diaphyseal femur fractures in bisphosphonate users compared with raloxifene/calcitonin users. However, this study cannot exclude the possibility that long-term bisphosphonate use may increase the risk of these fractures.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures


References
-
- Guyatt G, Cranney A, Griffith L, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am. 2002;31:659–679. - PubMed
-
- Kwek E, Koh J, Howe T. More on atypical fractures of the femoral diaphysis. N Engl J Med. 2008;359:316–317. - PubMed
-
- Lee P, Seibel M. More on atypical fractures of the femoral diaphysis. N Engl J Med. 2008;359:317. - PubMed
-
- Edwards M, McCrae F, Young-Min S. Alendronate-related femoral diaphysis fracture: what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporos Int. 2010;21:701–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

