Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase
- PMID: 21542014
- PMCID: PMC3179303
- DOI: 10.1002/jbmr.298
Effects of 25-hydroxyvitamin D(3) on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase
Abstract
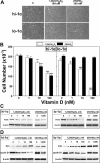
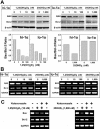
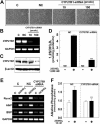
1,25-Dihydroxyvitamin D(3)[1,25(OH)(2)D(3)] has many noncalcemic actions that rest on inhibition of proliferation and promotion of differentiation in malignant and normal cell types. 1,25(OH)(2)D(3) stimulates osteoblast differentiation of human marrow stromal cells (hMSCs), but little is known about the effects of 25-hydroxyvitamin D(3)[25(OH)D(3)] on these cells. Recent evidence shows that hMSCs participate in vitamin D metabolism and can activate 25(OH)D(3) by CYP27B1/1α-hydroxylase. These studies test the hypothesis that antiproliferative and prodifferentiation effects of 25(OH)D(3) in hMSCs depend on CYP27B1. We studied hMSCs that constitutively express high (hMSCs(hi-1α) ) or low (hMSCs(lo-1α)) levels of CYP27B1 with equivalent expression of CYP24A1 and vitamin D receptor. In hMSCs(hi-1α), 25(OH)D(3) reduced proliferation, downregulated proliferating cell nuclear antigen (PCNA), upregulated p21(Waf1/Cip1), and decreased cyclin D1. Unlike 1,25(OH)(2)D(3), the antiapoptotic effects of 25(OH)D(3) on Bax and Bcl-2 were blocked by the P450 inhibitor ketoconazole. The antiproliferative effects of 25(OH)D(3) in hMSCs(hi-1α) and of 1,25(OH)(2)D(3) in both samples of hMSCs were explained by cell cycle arrest, not by increased apoptosis. Stimulation of osteoblast differentiation in hMSCs(hi-1α) by 25(OH)D(3) was prevented by ketoconazole and upon transfection with CYP27B1 siRNA. These data indicate that CYP27B1 is required for 25(OH)D(3)'s action in hMSCs. Three lines of evidence indicate that CYP27B1 is required for the antiproliferative and prodifferentiation effects of 25(OH)D(3) on hMSCs: Those effects were not seen (1) in hMSCs with low constitutive expression of CYP27B1, (2) in hMSCs treated with ketoconazole, and (3) in hMSCs in which CYP27B1 expression was silenced. Osteoblast differentiation and skeletal homeostasis may be regulated by autocrine/paracrine actions of 25(OH)D(3) in hMSCs.
Copyright © 2011 American Society for Bone and Mineral Research.
Figures


Similar articles
-
Vitamin D metabolism and action in human bone marrow stromal cells.Endocrinology. 2010 Jan;151(1):14-22. doi: 10.1210/en.2009-0969. Epub 2009 Dec 4. Endocrinology. 2010. PMID: 19966181 Free PMC article.
-
Age-related decline in osteoblastogenesis and 1α-hydroxylase/CYP27B1 in human mesenchymal stem cells: stimulation by parathyroid hormone.Aging Cell. 2011 Dec;10(6):962-71. doi: 10.1111/j.1474-9726.2011.00735.x. Epub 2011 Aug 24. Aging Cell. 2011. PMID: 21824271 Free PMC article.
-
Vitamin D metabolism and regulation in pediatric MSCs.J Steroid Biochem Mol Biol. 2016 Nov;164:287-291. doi: 10.1016/j.jsbmb.2015.09.025. Epub 2015 Sep 15. J Steroid Biochem Mol Biol. 2016. PMID: 26385609
-
Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells.Metabolism. 2013 Jun;62(6):768-77. doi: 10.1016/j.metabol.2013.01.003. Epub 2013 Jan 30. Metabolism. 2013. PMID: 23375059 Free PMC article. Review.
-
Metabolism of vitamin D3 by cytochromes P450.Front Biosci. 2005 Jan 1;10:119-34. doi: 10.2741/1514. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574355 Review.
Cited by
-
Extrahepatic 25-Hydroxylation of Vitamin D3 in an Engineered Osteoblast Precursor Cell Line Exploring the Influence on Cellular Proliferation and Matrix Maturation during Bone Development.ISRN Biomed Eng. 2013;2013:956362. doi: 10.1155/2013/956362. Epub 2013 Jun 4. ISRN Biomed Eng. 2013. PMID: 34909434 Free PMC article.
-
Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells.J Bone Miner Res. 2012 Sep;27(9):1992-2000. doi: 10.1002/jbmr.1655. J Bone Miner Res. 2012. PMID: 22576852 Free PMC article.
-
25-Hydroxyvitamin D(3)-loaded PLA microspheres: in vitro characterization and application in diabetic periodontitis models.AAPS PharmSciTech. 2013 Jun;14(2):880-9. doi: 10.1208/s12249-013-9978-5. Epub 2013 May 8. AAPS PharmSciTech. 2013. PMID: 23653087 Free PMC article.
-
Histone deacetylation mediates the rejuvenation of osteoblastogenesis by the combination of 25(OH)D3 and parathyroid hormone in MSCs from elders.J Steroid Biochem Mol Biol. 2013 Jul;136:156-9. doi: 10.1016/j.jsbmb.2012.09.002. Epub 2012 Sep 12. J Steroid Biochem Mol Biol. 2013. PMID: 22982627 Free PMC article.
-
Effects of Supplemental Vitamin D on Bone Health Outcomes in Women and Men in the VITamin D and OmegA-3 TriaL (VITAL).J Bone Miner Res. 2020 May;35(5):883-893. doi: 10.1002/jbmr.3958. Epub 2020 Jan 30. J Bone Miner Res. 2020. PMID: 31923341 Free PMC article. Clinical Trial.
References
-
- DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. - PubMed
-
- Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. - PubMed
-
- Verlinden L, Verstuyf A, Convents R, Marcelis S, Van Camp M, Bouillon R. Action of 1,25(OH)2D3 on the cell cycle genes cyclin D1, p21, and p27 in MCF-7 cells. Mol Cell Endocrinol. 1998;142:57–65. - PubMed
-
- Vandewalle B, Wattez N, Lefebvre J. Effects of vitamin D3 derivatives on growth, differentiation and apoptosis in tumoral colonic HT 29 cells: possible implication of intracellular calcium. Cancer Lett. 1995;97:99–106. - PubMed
-
- Ylikomi T, Laaksi I, Lou YR, et al. Antiproliferative action of vitamin D. Vitam Horm. 2002;64:357–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

