Enantioselective dichlorination of allylic alcohols
- PMID: 21542622
- PMCID: PMC3107004
- DOI: 10.1021/ja202555m
Enantioselective dichlorination of allylic alcohols
Abstract
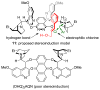
The development of an enantioselective allylic alcohol dichlorination catalyzed by dimeric cinchona alkaloid derivatives and employing aryl iododichlorides as chlorine sources is reported. Reaction optimization, exploration of the substrate scope, and a model for stereoinduction are presented.
© 2011 American Chemical Society
Figures



References
-
-
Enantioselective approaches: Julia S, Ginebreda A. Tetrahedron Lett. 1979;20:2171.Adam W, Mock-Knoblauch C, Saha-Möller CR, Herderich M. J Am Chem Soc. 2000;122:9685.Snyder SA, Tang ZY, Gupta R. J Am Chem Soc. 2009;131:5744.Snyder SA, Treitler DS, Brucks AP. J Am Chem Soc. 2010;132:14303.Diastereospecific approaches: Iranpoor N, Firouzabadi H, Azadi R, Ebrahimzadeh F. Can J Chem. 2006;84:69.Yoshimitsu T, Fukumoto N, Tanaka T. J Org Chem. 2009;74:696.Denton RM, Tang X, Przeslak A. Org Lett. 2010;12:4678.Diastereoselective approach: Shibuya GM, Kanady JS, Vanderwal CD. J Am Chem Soc. 2008;130:12514.
-
-
- Gribble GW. Acc Chem Res. 1998;31:141.
- Kladi M, Vagias C, Roussis V. Phytochem Rev. 2004;3:337.
-
- Ciminiello P, Fattorusso E, Forino M, Di Rosa M, Ianaro A, Poletti R. J Org Chem. 2001;66:578. - PubMed
-
- Mooney CL, Haines TH. Biochemistry. 1973;12:4469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

