Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium
- PMID: 21542647
- PMCID: PMC6309322
- DOI: 10.1021/mp2001704
Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium
Abstract
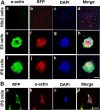
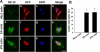
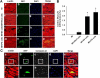
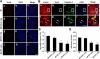
Cardiac myocyte differentiation reported thus far is from iPS cells generated from mouse and human fibroblasts. However, there is no article on the generation of iPS cells from cardiac ventricular specific cell types such as H9c2 cells. Therefore, whether transduced H9c2 cells, originally isolated from embryonic cardiac ventricular tissue, will be able to generate iPS cells and have the potential to repair and regenerate infarcted myocardium remains completely elusive. We transduced H9c2 cells with four stemness factors, Oct3/4, Sox2, Klf4, and c-Myc, and successfully reprogrammed them into iPS cells. These iPS cells were able to differentiate into beating cardiac myocytes and positively stained for cardiac specific sarcomeric α-actin and myosin heavy chain proteins. Following transplantation in the infarcted myocardium, there were newly differentiated cardiac myocytes and formation of gap junction proteins at 2 weeks post-myocardial infarction (MI), suggesting newly formed cardiac myocytes were integrated into the native myocardium. Furthermore, transplanted iPS cells significantly (p < 0.05) inhibited apoptosis and fibrosis and improved cardiac function compared with MI and MI+H9c2 cell groups. Moreover, our iPS cell derived cardiac myocyte differentiation in vitro and in vivo was comparable to embryonic stem cells in the present study. In conclusion we report for the first time that we have H9c2 cell-derived iPS cells which contain the potential to differentiate into cardiac myocytes in the cell culture system and repair and regenerate infarcted myocardium with improved cardiac function in vivo.
Figures

References
-
- Anversa P, Olivetti G, Leri A, Liu Y, Kajstura J. Myocyte cell death and ventricular remodeling. Curr. Opin. Nephrol. Hypertens. 1997;6:169–176. - PubMed
-
- Haider HK, Ashraf M. Bone marrow cell transplantation in clinical perspective. J. Mol. Cell Cardiol. 2005;38:225–235. - PubMed
-
- Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ. Res. 2005;96:127–137. - PubMed
-
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

