Growth factor priming of synovium-derived stem cells for cartilage tissue engineering
- PMID: 21542714
- PMCID: PMC3161099
- DOI: 10.1089/ten.TEA.2011.0155
Growth factor priming of synovium-derived stem cells for cartilage tissue engineering
Abstract
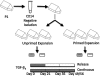
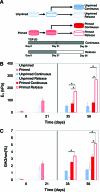
This study investigated the potential use of synovium-derived stem cells (SDSCs) as a cell source for cartilage tissue engineering. Harvested SDSCs from juvenile bovine synovium were expanded in culture in the presence (primed) or absence (unprimed) of growth factors (1 ng/mL transforming growth factor-β(1), 10 ng/mL platelet-derived growth factor-ββ, and 5 ng/mL basic fibroblast growth factor-2) and subsequently seeded into clinically relevant agarose hydrogel scaffolds. Constructs seeded with growth factor-primed SDSCs that received an additional transient application of transforming growth factor-β(3) for the first 21 days (release) exhibited significantly better mechanical and biochemical properties compared to constructs that received sustained growth factor stimulation over the entire culture period (continuous). In particular, the release group exhibited a Young's modulus (267±96 kPa) approaching native immature bovine cartilage levels, with corresponding glycosaminoglycan content (5.19±1.45%ww) similar to native values, within 7 weeks of culture. These findings suggest that SDSCs may serve as a cell source for cartilage tissue engineering applications.
Figures





References
-
- Guilak F. Sah R.L. Setton L.A. Physical regulation of cartilage metabolism. In: Mow V.C., editor; Hayes W.C., editor. Basic Orthopaedic Biomechanics. New York: Raven Press; 1997. pp. 197–207.
-
- Mow V.C. Bachrach N.M. Setton L.A. Guilak F. Stress, strain, pressure, and flow fields in articular cartilage and chondrocytes. In: Mow V.C., editor; Guilak F., editor; Hayes W.C., editor. Cell Mechanics and Cellular Engineering. New York: Springer-Verlag; 1994. pp. 345–379.
-
- Ronziere M.C. Perrier E. Mallein-Gerin F. Freyria A.M. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng. 2010;20:145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

