Interspecies chimera between primate embryonic stem cells and mouse embryos: monkey ESCs engraft into mouse embryos, but not post-implantation fetuses
- PMID: 21543277
- PMCID: PMC5053765
- DOI: 10.1016/j.scr.2011.03.002
Interspecies chimera between primate embryonic stem cells and mouse embryos: monkey ESCs engraft into mouse embryos, but not post-implantation fetuses
Abstract
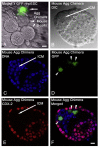
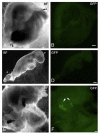
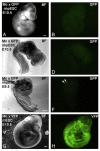
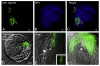
Unequivocal evidence for pluripotency in which embryonic stem cells contribute to chimeric offspring has yet to be demonstrated in human or nonhuman primates (NHPs). Here, rhesus and baboons ESCs were investigated in interspecific mouse chimera generated by aggregation or blastocyst injection. Aggregation chimera produced mouse blastocysts with GFP-nhpESCs at the inner cell mass (ICM), and embryo transfers (ETs) generated dimly-fluorescencing abnormal fetuses. Direct injection of GFP-nhpESCs into blastocysts produced normal non-GFP-fluorescencing fetuses. Injected chimera showed >70% loss of GFP-nhpESCs after 21 h culture. Outgrowths of all chimeric blastocysts established distinct but separate mouse- and NHP-ESC colonies. Extensive endogenous autofluorescence compromised anti-GFP detection and PCR analysis did not detect nhpESCs in fetuses. NhpESCs localize to the ICM in chimera and generate pregnancies. Because primate ESCs do not engraft post-implantation, and also because endogenous autofluorescence results in misleading positive signals, interspecific chimera assays for pluripotency with primate stem cells is unreliable with the currently available ESCs. Testing primate ESCs reprogrammed into even more naïve states in these inter-specific chimera assays will be an important future endeavor.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Domesticated cynomolgus monkey embryonic stem cells allow the generation of neonatal interspecies chimeric pigs.Protein Cell. 2020 Feb;11(2):97-107. doi: 10.1007/s13238-019-00676-8. Epub 2019 Nov 28. Protein Cell. 2020. PMID: 31781970 Free PMC article.
-
Generation of chimeric rhesus monkeys.Cell. 2012 Jan 20;148(1-2):285-95. doi: 10.1016/j.cell.2011.12.007. Epub 2012 Jan 5. Cell. 2012. PMID: 22225614 Free PMC article.
-
Apoptosis, G1 Phase Stall, and Premature Differentiation Account for Low Chimeric Competence of Human and Rhesus Monkey Naive Pluripotent Stem Cells.Stem Cell Reports. 2021 Jan 12;16(1):56-74. doi: 10.1016/j.stemcr.2020.12.004. Epub 2020 Dec 30. Stem Cell Reports. 2021. PMID: 33382978 Free PMC article.
-
Primate embryogenesis predicts the hallmarks of human naïve pluripotency.Development. 2017 Jan 15;144(2):175-186. doi: 10.1242/dev.145177. Development. 2017. PMID: 28096211 Free PMC article. Review.
-
Stem cell potency and the ability to contribute to chimeric organisms.Reproduction. 2013 Mar 7;145(3):R81-8. doi: 10.1530/REP-12-0396. Print 2013 Mar 1. Reproduction. 2013. PMID: 23221011 Free PMC article. Review.
Cited by
-
An expedition in the jungle of pluripotent stem cells of non-human primates.Stem Cell Reports. 2023 Nov 14;18(11):2016-2037. doi: 10.1016/j.stemcr.2023.09.013. Epub 2023 Oct 19. Stem Cell Reports. 2023. PMID: 37863046 Free PMC article. Review.
-
Stem cells and interspecies chimaeras.Nature. 2016 Dec 1;540(7631):51-59. doi: 10.1038/nature20573. Nature. 2016. PMID: 27905428 Review.
-
Human-animal chimeras for autologous organ transplantation: technological advances and future perspectives.Ann Transl Med. 2019 Oct;7(20):576. doi: 10.21037/atm.2019.10.13. Ann Transl Med. 2019. PMID: 31807557 Free PMC article. Review.
-
Interordinal chimera formation between medaka and zebrafish for analyzing stem cell differentiation.Stem Cells Dev. 2012 Aug 10;21(12):2333-41. doi: 10.1089/scd.2011.0630. Epub 2012 Feb 7. Stem Cells Dev. 2012. PMID: 22204449 Free PMC article.
-
Capturing Human Naïve Pluripotency in the Embryo and in the Dish.Stem Cells Dev. 2017 Aug 15;26(16):1141-1161. doi: 10.1089/scd.2017.0055. Epub 2017 Jun 26. Stem Cells Dev. 2017. PMID: 28537488 Free PMC article. Review.
References
-
- Ahrens ET, Srinivas M, Capuano S, Simhan HN, Schatten GP. Magnetic resonance imaging of embryonic and fetal development in model systems. Methods Mol. Med. 2006;124:87–101. - PubMed
-
- Behringer RR. Human–animal chimeras in biomedical research. Cell Stem Cell. 2007;1:259–262. - PubMed
-
- Boyd AS, Wu DC, Higashi Y, Wood KJ. A comparison of protocols used to generate insulin-producing cell clusters from mouse embryonic stem cells. Stem Cells. 2008;26:1128–1137. - PubMed
-
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, de Sousa Lopes S.M. Chuva, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

