Protein-disulfide isomerase displaces the cholera toxin A1 subunit from the holotoxin without unfolding the A1 subunit
- PMID: 21543321
- PMCID: PMC3121353
- DOI: 10.1074/jbc.M111.237966
Protein-disulfide isomerase displaces the cholera toxin A1 subunit from the holotoxin without unfolding the A1 subunit
Abstract
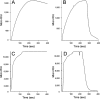
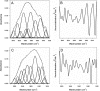
Protein-disulfide isomerase (PDI) has been proposed to exhibit an "unfoldase" activity against the catalytic A1 subunit of cholera toxin (CT). Unfolding of the CTA1 subunit is thought to displace it from the CT holotoxin and to prepare it for translocation to the cytosol. To date, the unfoldase activity of PDI has not been demonstrated for any substrate other than CTA1. An alternative explanation for the putative unfoldase activity of PDI has been suggested by recent structural studies demonstrating that CTA1 will unfold spontaneously upon its separation from the holotoxin at physiological temperature. Thus, PDI may simply dislodge CTA1 from the CT holotoxin without unfolding the CTA1 subunit. To evaluate the role of PDI in CT disassembly and CTA1 unfolding, we utilized a real-time assay to monitor the PDI-mediated separation of CTA1 from the CT holotoxin and directly examined the impact of PDI binding on CTA1 structure by isotope-edited Fourier transform infrared spectroscopy. Our collective data demonstrate that PDI is required for disassembly of the CT holotoxin but does not unfold the CTA1 subunit, thus uncovering a new mechanism for CTA1 dissociation from its holotoxin.
Figures
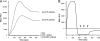


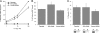
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

