N-formyl peptide receptor 3 (FPR3) departs from the homologous FPR2/ALX receptor with regard to the major processes governing chemoattractant receptor regulation, expression at the cell surface, and phosphorylation
- PMID: 21543323
- PMCID: PMC3143635
- DOI: 10.1074/jbc.M111.244590
N-formyl peptide receptor 3 (FPR3) departs from the homologous FPR2/ALX receptor with regard to the major processes governing chemoattractant receptor regulation, expression at the cell surface, and phosphorylation
Abstract
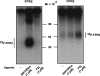
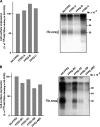
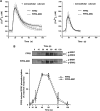
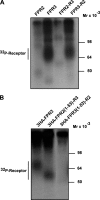
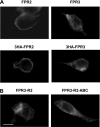
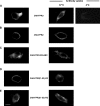
Among human N-formyl peptide chemoattractant receptors, FPR2/ALX and FPR3 share the highest degree of amino acid identity (83%), and trigger similar cell responses upon ligand binding. Although FPR2/ALX is a promiscuous receptor, FPR3 has only one specific high affinity ligand, F2L, and a more restricted tissue/cell distribution. In this study, we showed that FPR2/ALX behaved as the prototypical receptor FPR1. The agonist-dependent phosphorylation used a hierarchical mechanism with a prominent role of Ser(329), Thr(332), and Thr(335). Phosphorylation of FPR2/ALX was essential for its desensitization but the lack of phosphorylation did not result in enhanced or sustained responses. In contrast, resting FPR3 displayed a marked level of phosphorylation, which was only slightly increased upon agonist stimulation. Another noticeable difference between the two receptors was their subcellular distribution in unstimulated cells. Although FPR2/ALX was evenly distributed at the plasma membrane FPR3 was localized in small intracellular vesicles. By swapping domains between FPR2/ALX and FPR3, we uncovered the determinants involved in the basal phosphorylation of FPR3. Experiments aimed at monitoring receptor-bound antibody uptake showed that the intracellular distribution of FPR3 resulted from a constitutive internalization that was independent of C terminus phosphorylation. Unexpectedly, exchanging residues 1 to 53, which encompass the N-terminal extracellular region and the first transmembrane domain, between FPR2/ALX and FPR3 switched localization of the receptors from the plasma membrane to intracellular vesicles and vice versa. A clathrin-independent, possibly caveolae-dependent, mechanism was involved in FPR3 constitutive internalization. The peculiar behavior of FPR3 most probably serves distinct physiological functions that remain largely unknown.
Figures




Similar articles
-
Annexin A1 interaction with the FPR2/ALX receptor: identification of distinct domains and downstream associated signaling.J Biol Chem. 2012 Jul 13;287(29):24690-7. doi: 10.1074/jbc.M112.377101. Epub 2012 May 18. J Biol Chem. 2012. PMID: 22610094 Free PMC article.
-
FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis.FASEB J. 2010 Nov;24(11):4240-9. doi: 10.1096/fj.10-159913. Epub 2010 Jun 22. FASEB J. 2010. PMID: 20570963 Free PMC article.
-
Heterologously expressed formyl peptide receptor 2 (FPR2/ALX) does not respond to lipoxin A₄.Biochem Pharmacol. 2013 Jun 15;85(12):1795-802. doi: 10.1016/j.bcp.2013.04.019. Epub 2013 May 1. Biochem Pharmacol. 2013. PMID: 23643932
-
Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways.Pharmacol Ther. 2013 Dec;140(3):280-9. doi: 10.1016/j.pharmthera.2013.07.007. Epub 2013 Jul 21. Pharmacol Ther. 2013. PMID: 23880288 Review.
-
International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family.Pharmacol Rev. 2009 Jun;61(2):119-61. doi: 10.1124/pr.109.001578. Epub 2009 Jun 4. Pharmacol Rev. 2009. PMID: 19498085 Free PMC article. Review.
Cited by
-
Identification of C-terminal phosphorylation sites of N-formyl peptide receptor-1 (FPR1) in human blood neutrophils.J Biol Chem. 2013 Sep 20;288(38):27042-27058. doi: 10.1074/jbc.M113.484113. Epub 2013 Jul 19. J Biol Chem. 2013. PMID: 23873933 Free PMC article.
-
The Contribution of Formyl Peptide Receptor Dysfunction to the Course of Neuroinflammation: A Potential Role in the Brain Pathology.Curr Neuropharmacol. 2020;18(3):229-249. doi: 10.2174/1570159X17666191019170244. Curr Neuropharmacol. 2020. PMID: 31629396 Free PMC article. Review.
-
Adaptive evolution of formyl peptide receptors in mammals.J Mol Evol. 2015 Feb;80(2):130-41. doi: 10.1007/s00239-015-9666-z. Epub 2015 Jan 28. J Mol Evol. 2015. PMID: 25627928
-
Regulation of inflammation by lipid mediators in oral diseases.Oral Dis. 2017 Jul;23(5):576-597. doi: 10.1111/odi.12544. Epub 2016 Aug 4. Oral Dis. 2017. PMID: 27426637 Free PMC article. Review.
-
NOX2-Dependent Reactive Oxygen Species Regulate Formyl-Peptide Receptor 1-Mediated TrkA Transactivation in SH-SY5Y Cells.Oxid Med Cell Longev. 2019 Dec 2;2019:2051235. doi: 10.1155/2019/2051235. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31871542 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

