Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition
- PMID: 21543325
- PMCID: PMC3137029
- DOI: 10.1074/jbc.M111.229013
Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition
Abstract
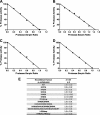
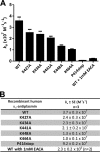
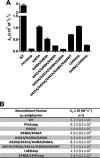
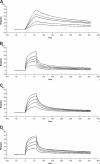
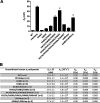
α(2)-Antiplasmin is the physiological inhibitor of plasmin and is unique in the serpin family due to N- and C-terminal extensions beyond its core domain. The C-terminal extension comprises 55 amino acids from Asn-410 to Lys-464, and the lysine residues (Lys-418, Lys-427, Lys-434, Lys-441, Lys-448, and Lys-464) within this region are important in mediating the initial interaction with kringle domains of plasmin. To understand the role of lysine residues within the C terminus of α(2)-antiplasmin, we systematically and sequentially mutated the C-terminal lysines, studied the effects on the rate of plasmin inhibition, and measured the binding affinity for plasmin via surface plasmon resonance. We determined that the C-terminal lysine (Lys-464) is individually most important in initiating binding to plasmin. Using two independent methods, we also showed that the conserved internal lysine residues play a major role mediating binding of the C terminus of α(2)-antiplasmin to kringle domains of plasmin and in accelerating the rate of interaction between α(2)-antiplasmin and plasmin. When the C terminus of α(2)-antiplasmin was removed, the binding affinity for active site-blocked plasmin remained high, suggesting additional exosite interactions between the serpin core and plasmin.
Figures

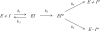
Similar articles
-
The human alpha(2)-plasmin inhibitor: functional characterization of the unique plasmin(ogen)-binding region.Cell Mol Life Sci. 2010 May;67(9):1505-18. doi: 10.1007/s00018-010-0264-3. Epub 2010 Jan 29. Cell Mol Life Sci. 2010. PMID: 20112045 Free PMC article.
-
Structural/functional characterization of the alpha 2-plasmin inhibitor C-terminal peptide.Biochemistry. 2003 Feb 4;42(4):1078-85. doi: 10.1021/bi026917n. Biochemistry. 2003. PMID: 12549929
-
Plasmin's peptide-binding specificity: characterization of ligand sites in alpha 2-antiplasmin.Thromb Res. 1989 Jun 15;54(6):621-32. doi: 10.1016/0049-3848(89)90128-x. Thromb Res. 1989. PMID: 2551057
-
Antiplasmin: the forgotten serpin?FEBS J. 2005 Oct;272(19):4852-7. doi: 10.1111/j.1742-4658.2005.04881.x. FEBS J. 2005. PMID: 16176259 Review.
-
Natural heterogeneity of α2-antiplasmin: functional and clinical consequences.Blood. 2016 Feb 4;127(5):538-45. doi: 10.1182/blood-2015-09-670117. Epub 2015 Dec 1. Blood. 2016. PMID: 26626994 Review.
Cited by
-
Increased urokinase and consumption of α2 -antiplasmin as an explanation for the loss of benefit of tranexamic acid after treatment delay.J Thromb Haemost. 2019 Jan;17(1):195-205. doi: 10.1111/jth.14338. Epub 2018 Dec 13. J Thromb Haemost. 2019. PMID: 30451372 Free PMC article.
-
α2-Antiplasmin as a potential regulator of the spatial memory process and age-related cognitive decline.Mol Brain. 2020 Oct 15;13(1):140. doi: 10.1186/s13041-020-00677-3. Mol Brain. 2020. PMID: 33059734 Free PMC article.
-
Plasminogen Receptors and Fibrinolysis.Int J Mol Sci. 2021 Feb 8;22(4):1712. doi: 10.3390/ijms22041712. Int J Mol Sci. 2021. PMID: 33567773 Free PMC article. Review.
-
A High-Throughput Small-Angle X-ray Scattering Assay to Determine the Conformational Change of Plasminogen.Int J Mol Sci. 2023 Sep 19;24(18):14258. doi: 10.3390/ijms241814258. Int J Mol Sci. 2023. PMID: 37762561 Free PMC article.
-
Alpha2-Antiplasmin: The Devil You Don't Know in Cerebrovascular and Cardiovascular Disease.Front Cardiovasc Med. 2020 Dec 23;7:608899. doi: 10.3389/fcvm.2020.608899. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 33426005 Free PMC article.
References
-
- Lijnen H. R. (2001) Ann. N.Y. Acad. Sci. 936, 226–236 - PubMed
-
- Matsuno H. (2006) Curr. Pharm. Des. 12, 841–847 - PubMed
-
- Gross P. L., Weitz J. I. (2009) Clin. Pharmacol. Ther. 86, 139–146 - PubMed
-
- Lee K. N., Lee C. S., Tae W. C., Jackson K. W., Christiansen V. J., McKee P. A. (2000) J. Biol. Chem. 275, 37382–37389 - PubMed
-
- Coughlin P. B. (2005) FEBS J. 272, 4852–4857 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

