Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition
- PMID: 21543325
- PMCID: PMC3137029
- DOI: 10.1074/jbc.M111.229013
Contribution of conserved lysine residues in the alpha2-antiplasmin C terminus to plasmin binding and inhibition
Abstract
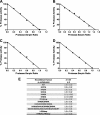
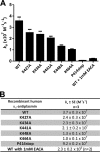
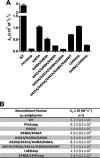
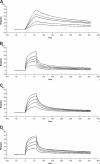
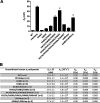
α(2)-Antiplasmin is the physiological inhibitor of plasmin and is unique in the serpin family due to N- and C-terminal extensions beyond its core domain. The C-terminal extension comprises 55 amino acids from Asn-410 to Lys-464, and the lysine residues (Lys-418, Lys-427, Lys-434, Lys-441, Lys-448, and Lys-464) within this region are important in mediating the initial interaction with kringle domains of plasmin. To understand the role of lysine residues within the C terminus of α(2)-antiplasmin, we systematically and sequentially mutated the C-terminal lysines, studied the effects on the rate of plasmin inhibition, and measured the binding affinity for plasmin via surface plasmon resonance. We determined that the C-terminal lysine (Lys-464) is individually most important in initiating binding to plasmin. Using two independent methods, we also showed that the conserved internal lysine residues play a major role mediating binding of the C terminus of α(2)-antiplasmin to kringle domains of plasmin and in accelerating the rate of interaction between α(2)-antiplasmin and plasmin. When the C terminus of α(2)-antiplasmin was removed, the binding affinity for active site-blocked plasmin remained high, suggesting additional exosite interactions between the serpin core and plasmin.
Figures

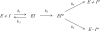
References
-
- Lijnen H. R. (2001) Ann. N.Y. Acad. Sci. 936, 226–236 - PubMed
-
- Matsuno H. (2006) Curr. Pharm. Des. 12, 841–847 - PubMed
-
- Gross P. L., Weitz J. I. (2009) Clin. Pharmacol. Ther. 86, 139–146 - PubMed
-
- Lee K. N., Lee C. S., Tae W. C., Jackson K. W., Christiansen V. J., McKee P. A. (2000) J. Biol. Chem. 275, 37382–37389 - PubMed
-
- Coughlin P. B. (2005) FEBS J. 272, 4852–4857 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

