Mitochondrial subversion in cancer
- PMID: 21543342
- PMCID: PMC3298745
- DOI: 10.1158/1940-6207.CAPR-10-0326
Mitochondrial subversion in cancer
Abstract
Mitochondria control essential cellular activities including generation of ATP via oxidative phosphorylation. Mitochondrial DNA (mtDNA) mutations in the regulatory D-loop region and somatic mtDNA mutations are common in primary human cancers. The biological impact of a given mutation may vary, depending on the nature of the mutation and the proportion of mutant mtDNAs carried by the cell. Identification of mtDNA mutations in precancerous lesions supports their early contribution to cell transformation and cancer progression. Introduction of mtDNA mutations in transformed cells has been associated with increased ROS production and tumor growth. Studies reveal that increased and altered mtDNA plays a role in the development of cancer but further work is required to establish the functional significance of specific mitochondrial mutations in cancer and disease progression. This review offers some insight into the extent of mtDNA mutations, their functional consequences in tumorigenesis, mitochondrial therapeutics, and future clinical application.
Figures

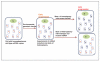
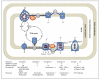
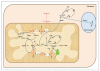

References
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. - PubMed
-
- Warburg O. Origin of cancer cells. Oncologia. 1956;9:75–83. - PubMed
-
- Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. - PubMed
-
- Lightowlers RN, Chinnery PF, Turnbull DM, Howell N. Mammalian mitochondrial genetics: heredity, heteroplasmy and disease. Trends Genet. 1997;13:450–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

