Adjuvant properties of a biocompatible thermo-responsive polymer of N-isopropylacrylamide in autoimmunity and arthritis
- PMID: 21543351
- PMCID: PMC3203480
- DOI: 10.1098/rsif.2011.0114
Adjuvant properties of a biocompatible thermo-responsive polymer of N-isopropylacrylamide in autoimmunity and arthritis
Abstract
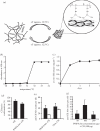
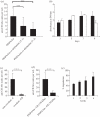
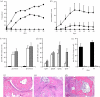
To evaluate the thermo-responsive poly(N-isopropylacrylamide) (PNiPAAm) polymer as an adjuvant, we synthesized PNiPAAm through free radical polymerization and characterized it both in vitro and in vivo. The polymer when mixed with collagen type II (CII) induced antigen-specific autoimmunity and arthritis. Mice immunized with PNiPAAm-CII developed significant levels of CII-specific IgG response comprising major IgG subclasses. Antigen-specific cellular recall response was also enhanced in these mice, while negligible level of IFN-γ was detected in splenocyte cultures, in vitro. PNiPAAm-CII-immunized arthritic mouse paws showed massive infiltration of immune cells and extensive damage to cartilage and bone. As determined by immunostaining, most of the CII protein retained its native configuration after injecting it with PNiPAAm in naive mice. Physical adsorption of CII and the high-molecular-weight form of moderately hydrophobic PNiPAAm induced a significant anti-CII antibody response. Similar to CII, mice immunized with PNiPAAm and ovalbumin (PNiPAAm-Ova) induced significant anti-ovalbumin antibody response. Comparable levels of serum IFN-γ, IL-1β and IL-17 were observed in ovalbumin-immunized mice with complete Freund, incomplete Freund (CFA and IFA) or PNiPAAm adjuvants. However, serum IL-4 levels were significantly higher in PNiPAAm-Ova and CFA-Ova groups compared with the IFA-Ova group. Thus, we show for the first time, biocompatible and biodegradable thermo-responsive PNiPAAm can be used as an adjuvant in several immunological applications as well as in better understanding of the autoimmune responses against self-proteins.
Figures


References
-
- Monach P. A., Benoist C., Mathis D. 2004. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv. Immunol. 82, 217–248 10.1016/S0065-2776(04)82005-4 (doi:10.1016/S0065-2776(04)82005-4) - DOI - PubMed
-
- Kannan K., Ortmann R. A., Kimpel D. 2005. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 12, 167–181 10.1016/j.pathophys.2005.07.011 (doi:10.1016/j.pathophys.2005.07.011) - DOI - PubMed
-
- Reed S. G., Bertholet S., Coler R. N., Friede M. 2009. New horizons in adjuvants for vaccine development. Trends Immunol. 30, 23–32 10.1016/j.it.2008.09.006 (doi:10.1016/j.it.2008.09.006) - DOI - PubMed
-
- Wilson-Welder J. H., Torres M. P., Kipper M. J., Mallapragada S. K., Wannemuehler M. J., Narasimhan B. 2009. Vaccine adjuvants: current challenges and future approaches. J. Pharm. Sci. 98, 1278–1316 10.1002/jps.21523 (doi:10.1002/jps.21523) - DOI - PMC - PubMed
-
- Edelman R. 2002. The development and use of vaccine adjuvants. Mol. Biotechnol. 21, 129–148 10.1385/MB:21:2:129 (doi:10.1385/MB:21:2:129) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
