Heliothis zea nudivirus 1 gene hhi1 induces apoptosis which is blocked by the Hz-iap2 gene and a noncoding gene, pag1
- PMID: 21543471
- PMCID: PMC3126586
- DOI: 10.1128/JVI.01843-10
Heliothis zea nudivirus 1 gene hhi1 induces apoptosis which is blocked by the Hz-iap2 gene and a noncoding gene, pag1
Abstract
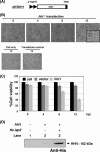
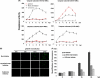
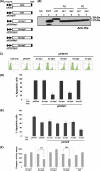
Heliothis zea nudivirus 1 (HzNV-1 or Hz-1 virus), previously regarded as a nonoccluded baculovirus, recently has been placed in the Nudivirus genus. This virus generates HzNV-1 HindIII-I 1 (hhi1) and many other transcripts during productive viral infection; during latent viral infection, however, persistency-associated gene 1 (pag1) is the only gene expressed. In this report, we used transient expression assays to show that hhi1 can trigger strong apoptosis in transfected cells, which can be blocked, at least partially, by the inhibitor of apoptosis genes Autographa californica iap2 (Ac-iap2) and H. zea iap2 (Hz-iap2). In addition to these two genes, unexpectedly, pag1, which encodes a noncoding RNA with no detectable protein product, was found to efficiently suppress hhi1-induced apoptosis. The assay of pro-Sf-caspase-1 processing by hhi1 transfection did not detect the small P12 subunit at any of the time intervals tested, suggesting that hhi1 of HzNV-1 induces apoptosis through alternative caspase pathways.
Figures




References
-
- Ahmad M., et al. 1997. Spodoptera frugiperda caspase-1, a novel insect death protease that cleaves the nuclear immunophilin FKBP46, is the target of the baculovirus antiapoptotic protein p35. J. Biol. Chem. 272:1421–1424 - PubMed
-
- Alimonti J. B., Ball T. B., Fowke K. R. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649–1661 - PubMed
-
- Ayres M. D., Howard S. C., Kuzio J., Lopez-Ferber M., Possee R. D. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586–605 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

