Adaptation of Saffold virus 2 for high-titer growth in mammalian cells
- PMID: 21543476
- PMCID: PMC3126574
- DOI: 10.1128/JVI.00265-11
Adaptation of Saffold virus 2 for high-titer growth in mammalian cells
Abstract
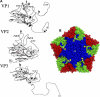
Saffold viruses (SAFV) are a recently discovered group of human Cardioviruses closely related to Theiler's murine encephalomyelitis viruses (TMEV). Unlike TMEV and encephalomyocarditis virus, each of which is monotypic, SAFV are genetically diverse and include at least eight genotypes. To date, only Saffold virus 3 (SAFV-3) has been grown efficiently in mammalian cells in vitro. Here, we report the successful adaptation of SAFV-2 for efficient growth in HeLa cells after 13 passages in the alpha/beta interferon-deficient human glial cell line U118 MG. Nine amino acid changes were found in the adapted virus, with single mutations in VP2, VP3, and 2B, while 6 mutations arose in VP1. Most capsid mutations were in surface loops. Analysis of SAFV-2 revealed virus growth and cytopathic effect only in human cell lines, with large plaques forming in HeLa cells, with minimal cell association, and without using sialic acid to enter cells. Despite the limited growth of SAFV-2 in rodent cells in vitro, BALB/c mice inoculated with SAFV-2 showed antibody titers of >1:10(6), and fluorescence-activated cell sorting (FACS) analysis revealed only minimal cross-reactivity with SFV-3. Intracerebral inoculation of 6-week-old FVB/n mice produced paralysis and acute neuropathological changes, including meningeal infiltrates, encephalitis, particularly of the limbic system, and spinal cord white matter inflammation.
Figures



Similar articles
-
Saffold virus, a human Theiler's-like cardiovirus, is ubiquitous and causes infection early in life.PLoS Pathog. 2009 May;5(5):e1000416. doi: 10.1371/journal.ppat.1000416. Epub 2009 May 1. PLoS Pathog. 2009. PMID: 19412527 Free PMC article.
-
Cultivation and serological characterization of a human Theiler's-like cardiovirus associated with diarrheal disease.J Virol. 2010 May;84(9):4407-14. doi: 10.1128/JVI.02536-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164225 Free PMC article.
-
The Pathogenesis of Saffold Virus in AG129 Mice and the Effects of Its Truncated L Protein in the Central Nervous System.Viruses. 2016 Feb 18;8(2):24. doi: 10.3390/v8020024. Viruses. 2016. PMID: 26901216 Free PMC article.
-
Saffold virus, a novel human Cardiovirus with unknown pathogenicity.J Virol. 2012 Feb;86(3):1292-6. doi: 10.1128/JVI.06087-11. Epub 2011 Nov 23. J Virol. 2012. PMID: 22114344 Free PMC article. Review.
-
Differential usage of carbohydrate co-receptors influences cellular tropism of Theiler's murine encephalomyelitis virus infection of the central nervous system.Glycoconj J. 2006 Feb;23(1-2):39-49. doi: 10.1007/s10719-006-5436-x. Glycoconj J. 2006. PMID: 16575521 Review.
Cited by
-
First Occurrence of Saffold Virus in Sewage and River Water Samples in Karaj, Iran.Food Environ Virol. 2020 Mar;12(1):75-80. doi: 10.1007/s12560-019-09415-y. Epub 2019 Nov 15. Food Environ Virol. 2020. PMID: 31729639
-
Saffold virus, an emerging human cardiovirus.Rev Med Virol. 2017 Jan;27(1):e1908. doi: 10.1002/rmv.1908. Epub 2016 Oct 10. Rev Med Virol. 2017. PMID: 27723176 Free PMC article. Review.
-
Saffold virus type 3 (SAFV-3) persists in HeLa cells.PLoS One. 2013;8(1):e53194. doi: 10.1371/journal.pone.0053194. Epub 2013 Jan 4. PLoS One. 2013. PMID: 23308162 Free PMC article.
-
Intracerebral Inoculation of Mouse-Passaged Saffold Virus Type 3 Affects Cerebellar Development in Neonatal Mice.J Virol. 2016 Oct 14;90(21):10007-10021. doi: 10.1128/JVI.00864-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27581974 Free PMC article.
-
Neuropathogenicity of Two Saffold Virus Type 3 Isolates in Mouse Models.PLoS One. 2016 Feb 1;11(2):e0148184. doi: 10.1371/journal.pone.0148184. eCollection 2016. PLoS One. 2016. PMID: 26828718 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

