Sprouty 2 binds ESCRT-II factor Eap20 and facilitates HIV-1 gag release
- PMID: 21543492
- PMCID: PMC3126580
- DOI: 10.1128/JVI.00141-11
Sprouty 2 binds ESCRT-II factor Eap20 and facilitates HIV-1 gag release
Abstract
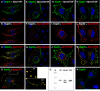
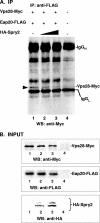
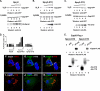
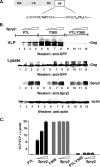
The four ESCRT (endocytic sorting complexes required for transport) complexes (ESCRT-0, -I, -II, and -III) normally operate sequentially in the trafficking of cellular cargo. HIV-1 Gag trafficking and release as virus-like particles (VLPs) require the participation of ESCRTs; however, its use of ESCRTs is selective and nonsequential. Specifically, Gag trafficking to release sites on the plasma membrane does not require ESCRT-0 or -II. It is known that a bypass of ESCRT-0 is achieved by the direct linkage of the ESCRT-I component, Tsg101, to the primary L domain motif (PTAP) in Gag and that bypass of ESCRT-II is achieved by the linkage of Gag to ESCRT-III through the adaptor protein Alix. However, the mechanism by which Gag suppresses the interaction of bound ESCRT-I with ESCRT-II is unknown. Here we show (i) that VLP release requires the steady-state level of Sprouty 2 (Spry2) in COS-1 cells, (ii) that Spry2 binds the ESCRT-II component Eap20, (iii) that binding Eap20 permits Spry2 to disrupt ESCRT-I interaction with ESCRT-II, and (iv) that coexpression of Gag with a Spry2 fragment that binds Eap20 increases VLP release. Spry2 also facilitated release of P7L-Gag (i.e., release in the absence of Tsg101 binding). In this case, rescue required the secondary L domain (YPX(n)L) in HIV-1 Gag that binds Alix and the region in Spry2 that binds Eap20. The results identify Spry2 as a novel cellular factor that facilitates release driven by the primary and secondary HIV-1 Gag L domains.
Figures



Similar articles
-
Avian sarcoma virus and human immunodeficiency virus, type 1 use different subsets of ESCRT proteins to facilitate the budding process.J Biol Chem. 2008 Oct 31;283(44):29822-30. doi: 10.1074/jbc.M804157200. Epub 2008 Aug 22. J Biol Chem. 2008. PMID: 18723511 Free PMC article.
-
Ubiquitin conjugation to Gag is essential for ESCRT-mediated HIV-1 budding.Retrovirology. 2013 Jul 29;10:79. doi: 10.1186/1742-4690-10-79. Retrovirology. 2013. PMID: 23895345 Free PMC article.
-
The functionally exchangeable L domains in RSV and HIV-1 Gag direct particle release through pathways linked by Tsg101.Traffic. 2005 Oct;6(10):880-94. doi: 10.1111/j.1600-0854.2005.00323.x. Traffic. 2005. PMID: 16138902 Free PMC article.
-
Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking.Viruses. 2021 Jun 15;13(6):1147. doi: 10.3390/v13061147. Viruses. 2021. PMID: 34203832 Free PMC article. Review.
-
Budding of a Retrovirus: Some Assemblies Required.Viruses. 2020 Oct 20;12(10):1188. doi: 10.3390/v12101188. Viruses. 2020. PMID: 33092109 Free PMC article. Review.
Cited by
-
Tsg101 regulates PI(4,5)P2/Ca(2+) signaling for HIV-1 Gag assembly.Front Microbiol. 2014 May 20;5:234. doi: 10.3389/fmicb.2014.00234. eCollection 2014. Front Microbiol. 2014. PMID: 24904548 Free PMC article.
-
HIV Assembly and Budding: Ca(2+) Signaling and Non-ESCRT Proteins Set the Stage.Mol Biol Int. 2012;2012:851670. doi: 10.1155/2012/851670. Epub 2012 Jun 12. Mol Biol Int. 2012. PMID: 22761998 Free PMC article.
-
Genomic tagging of endogenous human ESCRT-I complex preserves ESCRT-mediated membrane-remodeling functions.J Biol Chem. 2019 Nov 1;294(44):16266-16281. doi: 10.1074/jbc.RA119.009372. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519756 Free PMC article.
-
Involvement of ESCRT-II in hepatitis B virus morphogenesis.PLoS One. 2014 Mar 10;9(3):e91279. doi: 10.1371/journal.pone.0091279. eCollection 2014. PLoS One. 2014. PMID: 24614091 Free PMC article.
-
Insights into the function of ESCRT and its role in enveloped virus infection.Front Microbiol. 2023 Oct 6;14:1261651. doi: 10.3389/fmicb.2023.1261651. eCollection 2023. Front Microbiol. 2023. PMID: 37869652 Free PMC article. Review.
References
-
- Carter C. A., Ehrlich L. S. 2008. Cell biology of HIV-1 infection of macrophages. Annu. Rev. Microbiol. 62:425–443 - PubMed
-
- Chen C., Vincent O., Jin J., Weisz O. A., Montelaro R. C. 2005. Functions of early (AP-2) and late (AIP1/ALIX) endocytic proteins in equine infectious anemia virus budding. J. Biol. Chem. 280:40474. - PubMed
-
- Chu H., Wang J. J., Spearman P. 2009. Human immunodeficiency virus type-1 gag and host vesicular trafficking pathways. Curr. Top. Microbiol. Immunol. 339:67–84 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

