Absence of XMRV retrovirus and other murine leukemia virus-related viruses in patients with chronic fatigue syndrome
- PMID: 21543496
- PMCID: PMC3126563
- DOI: 10.1128/JVI.00693-11
Absence of XMRV retrovirus and other murine leukemia virus-related viruses in patients with chronic fatigue syndrome
Abstract
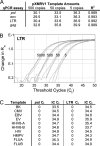
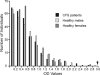
Chronic fatigue syndrome (CFS) is a multisystem disorder characterized by prolonged and severe fatigue that is not relieved by rest. Attempts to treat CFS have been largely ineffective primarily because the etiology of the disorder is unknown. Recently, CFS has been associated with xenotropic murine leukemia virus-related virus (XMRV) as well as other murine leukemia virus (MLV)-related viruses, though not all studies have found these associations. We collected blood samples from 100 CFS patients and 200 self-reported healthy volunteers from the same geographical area. We analyzed these in a blind manner using molecular, serological, and viral replication assays. We also analyzed samples from patients in the original study that reported XMRV in CFS patients. We did not find XMRV or related MLVs either as viral sequences or infectious viruses, nor did we find antibodies to these viruses in any of the patient samples, including those from the original study. We show that at least some of the discrepancy with previous studies is due to the presence of trace amounts of mouse DNA in the Taq polymerase enzymes used in these previous studies. Our findings do not support an association between CFS and MLV-related viruses, including XMRV, and the off-label use of antiretrovirals for the treatment of CFS does not seem justified at present.
Figures



References
-
- Carruthers B. M., et al. 2003. Myalgic encephalomyelitis/chronic fatigue syndrome. J. Chronic Fatigue Syndr. 11:7–115
-
- Devanur L. D., Kerr J. R. 2006. Chronic fatigue syndrome. J. Clin. Virol. 37:139–150 - PubMed
-
- Fukuda K., et al. 1994. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 121:953–959 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

