Acyl substrate preferences of an IAA-amido synthetase account for variations in grape (Vitis vinifera L.) berry ripening caused by different auxinic compounds indicating the importance of auxin conjugation in plant development
- PMID: 21543520
- PMCID: PMC3153680
- DOI: 10.1093/jxb/err134
Acyl substrate preferences of an IAA-amido synthetase account for variations in grape (Vitis vinifera L.) berry ripening caused by different auxinic compounds indicating the importance of auxin conjugation in plant development
Abstract
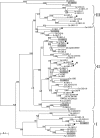
Nine Gretchen Hagen (GH3) genes were identified in grapevine (Vitis vinifera L.) and six of these were predicted on the basis of protein sequence similarity to act as indole-3-acetic acid (IAA)-amido synthetases. The activity of these enzymes is thought to be important in controlling free IAA levels and one auxin-inducible grapevine GH3 protein, GH3-1, has previously been implicated in the berry ripening process. Ex planta assays showed that the expression of only one other GH3 gene, GH3-2, increased following the treatment of grape berries with auxinic compounds. One of these was the naturally occurring IAA and the other two were synthetic, α-naphthalene acetic acid (NAA) and benzothiazole-2-oxyacetic acid (BTOA). The determination of steady-state kinetic parameters for the recombinant GH3-1 and GH3-2 proteins revealed that both enzymes efficiently conjugated aspartic acid (Asp) to IAA and less well to NAA, while BTOA was a poor substrate. GH3-2 gene expression was induced by IAA treatment of pre-ripening berries with an associated increase in levels of IAA-Asp and a decrease in free IAA levels. This indicates that GH3-2 responded to excess auxin to maintain low levels of free IAA. Grape berry ripening was not affected by IAA application prior to veraison (ripening onset) but was considerably delayed by NAA and even more so by BTOA. The differential effects of the three auxinic compounds on berry ripening can therefore be explained by the induction and acyl substrate specificity of GH3-2. These results further indicate an important role for GH3 proteins in controlling auxin-related plant developmental processes.
Figures





References
-
- Abbas MF, Abbas MJ, Abdel-Basit OI. Indole-3-acetic acid concentration during fruit development in date palm (Phoenix dactylifera L. cv. Hillawi) Fruit. 2000;55:115–118.
-
- Agustí M, Gariglio N, Castillo A, Juan M, Almela V, Martínez-Fuentes A, Mesejo C. Effect of the synthetic auxin 2,4-DP on fruit development of loquat. Journal of Plant Growth Regulation. 2003;41:129–132.
-
- Agustí M, Juan M, Mesejo C, Martínez-Fuentes A, Almela V. Persimmon fruit size and climacteric encouraged by 3,5,6-trichloro-2-pyridyloxyacetic acid. The Journal of Horticultural Science and Biotechnology. 2004;79:171–174.
-
- Aharoni A, Keizer LCP, Van den Broeck HC, Blanco-Portales R, Muñoz-Blanco J, Bois G, Smit P, De Vos RCH, O'Connell AP. Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a non-climacteric fruit. Plant Physiology. 2002;129:1019–1031. - PMC - PubMed

