Structural and functional analysis of two new positive allosteric modulators of GluA2 desensitization and deactivation
- PMID: 21543522
- PMCID: PMC3141890
- DOI: 10.1124/mol.110.070243
Structural and functional analysis of two new positive allosteric modulators of GluA2 desensitization and deactivation
Abstract
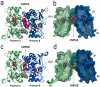
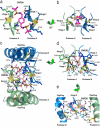
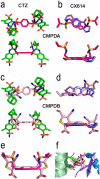
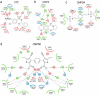
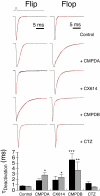
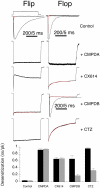
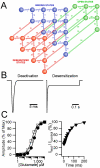
At the dimer interface of the extracellular ligand-binding domain of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors a hydrophilic pocket is formed that is known to interact with two classes of positive allosteric modulators, represented by cyclothiazide and the ampakine 2H,3H,6aH-pyrrolidino(2,1-3',2')1,3-oxazino(6',5'-5,4)benzo(e)1,4-dioxan-10-one (CX614). Here, we present structural and functional data on two new positive allosteric modulators of AMPA receptors, phenyl-1,4-bis-alkylsulfonamide (CMPDA) and phenyl-1,4-bis-carboxythiophene (CMPDB). Crystallographic data show that these compounds bind within the modulator-binding pocket and that substituents of each compound overlap with distinct moieties of cyclothiazide and CX614. The goals of the present study were to determine 1) the degree of modulation by CMPDA and CMPDB of AMPA receptor deactivation and desensitization; 2) whether these compounds are splice isoform-selective; and 3) whether predictions of mechanism of action could be inferred by comparing molecular interactions between the ligand-binding domain and each compound with those of cyclothiazide and CX614. CMPDB was found to be more isoform-selective than would be predicted from initial binding assays. It is noteworthy that these new compounds are both more potent and more effective and may be more clinically relevant than the AMPA receptor modulators described previously.
Figures


References
-
- Arai A, Kessler M, Ambros-Ingerson J, Quan A, Yigiter E, Rogers G, Lynch G. (1996a) Effects of a centrally active benzoylpyrrolidine drug on AMPA receptor kinetics. Neuroscience 75:573–585 - PubMed
-
- Arai A, Kessler M, Rogers G, Lynch G. (1996b) Effects of a memory-enhancing drug on DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther 278:627–638 - PubMed
-
- Arai AC, Kessler M, Rogers G, Lynch G. (2000) Effects of the potent ampakine CX614 on hippocampal and recombinant AMPA receptors: interactions with cyclothiazide and GYKI 52466. Mol Pharmacol 58:802–813 - PubMed
-
- Arai AC, Xia YF, Rogers G, Lynch G, Kessler M. (2002) Benzamide-type AMPA receptor modulators form two subfamilies with distinct modes of action. J Pharmacol Exp Ther 303:1075–1085 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

