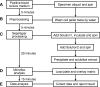
Improved identification of yeast species directly from positive blood culture media by combining Sepsityper specimen processing and Microflex analysis with the matrix-assisted laser desorption ionization Biotyper system
- PMID: 21543564
- PMCID: PMC3147835
- DOI: 10.1128/JCM.00339-11
Improved identification of yeast species directly from positive blood culture media by combining Sepsityper specimen processing and Microflex analysis with the matrix-assisted laser desorption ionization Biotyper system
Abstract
Current methods for identification of yeast from blood cultures may take several days after these microorganisms have been observed by Gram stain smears from positive blood cultures. We explored the use of a matrix-assisted laser desorption ionization (MALDI) Biotyper system in combination with Sepsityper specimen processing and Microflex analysis for improved detection and identification of yeast species directly from positive blood culture specimens demonstrating yeast-like organisms by Gram stain. The limit of detection of yeast species in blood culture medium was determined to be 5.9 × 10(5) CFU, with intra- and interstrain coefficients of variation of 1.8 to 3.6% and 2.9%, respectively. A total of 42 yeast-containing positive blood culture specimens were processed, and the identification results were compared to those obtained by routinely used phenotypic methods. Specimens with discrepant results between the Biotyper and phenotypic methods were identified on the basis of internal transcribed spacer region sequencing. The MALDI Biotyper system correctly identified the 42 specimens to species level, including 28 (66.7%) Candida albicans, 8 (19.0%) Candida parapsilosis, and 5 (11.9%) Candida tropicalis isolates and 1 (2.4%) Cryptococcus neoformans isolate. The entire procedure, from specimen extraction to final result reporting, can be completed within 1 h. Our data indicated that the Sepsityper specimen processing and Microflex analysis by the MALDI Biotyper system provide a rapid and reliable tool for yeast species identification directly from positive blood culture media.
Figures
Similar articles
-
Comparison between the Biflex III-Biotyper and the Axima-SARAMIS systems for yeast identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry.J Clin Microbiol. 2013 Apr;51(4):1231-6. doi: 10.1128/JCM.03268-12. Epub 2013 Feb 6. J Clin Microbiol. 2013. PMID: 23390281 Free PMC article.
-
Comparative evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) systems for non-albicans Candida and uncommon yeast isolates.J Microbiol Methods. 2021 Jun;185:106232. doi: 10.1016/j.mimet.2021.106232. Epub 2021 May 4. J Microbiol Methods. 2021. PMID: 33961963
-
Comparison of the MALDI Biotyper system using Sepsityper specimen processing to routine microbiological methods for identification of bacteria from positive blood culture bottles.J Clin Microbiol. 2012 Feb;50(2):346-52. doi: 10.1128/JCM.05021-11. Epub 2011 Dec 7. J Clin Microbiol. 2012. PMID: 22162549 Free PMC article.
-
Comparison of the Bruker Biotyper and VITEK MS Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry Systems Using a Formic Acid Extraction Method to Identify Common and Uncommon Yeast Isolates.Ann Lab Med. 2017 May;37(3):223-230. doi: 10.3343/alm.2017.37.3.223. Ann Lab Med. 2017. PMID: 28224768 Free PMC article.
-
Multicenter study evaluating the Vitek MS system for identification of medically important yeasts.J Clin Microbiol. 2013 Jul;51(7):2267-72. doi: 10.1128/JCM.00680-13. Epub 2013 May 8. J Clin Microbiol. 2013. PMID: 23658267 Free PMC article.
Cited by
-
A differential centrifugation protocol and validation criterion for enhancing mass spectrometry (MALDI-TOF) results in microbial identification using blood culture growth bottles.Eur J Clin Microbiol Infect Dis. 2013 May;32(5):699-704. doi: 10.1007/s10096-012-1797-1. Epub 2012 Dec 30. Eur J Clin Microbiol Infect Dis. 2013. PMID: 23274860
-
Rapid identification of pathogens in positive blood culture of patients with sepsis: review and meta-analysis of the performance of the sepsityper kit.Int J Microbiol. 2015;2015:827416. doi: 10.1155/2015/827416. Epub 2015 Apr 27. Int J Microbiol. 2015. PMID: 26000017 Free PMC article. Review.
-
Rapid direct identification of positive paediatric blood cultures by MALDI-TOF MS technology and its clinical impact in the paediatric hospital setting.BMC Res Notes. 2020 Jan 6;13(1):12. doi: 10.1186/s13104-019-4861-4. BMC Res Notes. 2020. PMID: 31907060 Free PMC article.
-
Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Use with Positive Blood Cultures: Methodology, Performance, and Optimization.J Clin Microbiol. 2017 Dec;55(12):3328-3338. doi: 10.1128/JCM.00868-17. Epub 2017 Aug 30. J Clin Microbiol. 2017. PMID: 28855303 Free PMC article. Review.
-
MALDI-TOF MS distinctly differentiates nontypable Haemophilus influenzae from Haemophilus haemolyticus.PLoS One. 2013;8(2):e56139. doi: 10.1371/journal.pone.0056139. Epub 2013 Feb 14. PLoS One. 2013. PMID: 23457514 Free PMC article.
References
-
- Alispahic M., et al. 2010. Species-specific identification and differentiation of Arcobacter, Helicobacter and Campylobacter by full-spectral matrix-associated laser desorption/ionization time of flight mass spectrometry analysis. J. Med. Microbiol. 59:295–301 - PubMed
-
- Bader O., et al. 14 October 2010. Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. [Epub ahead of print.] - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases