Study of novel selective mGlu2 agonist in the temporo-ammonic input to CA1 neurons reveals reduced mGlu2 receptor expression in a Wistar substrain with an anxiety-like phenotype
- PMID: 21543601
- PMCID: PMC6632860
- DOI: 10.1523/JNEUROSCI.0418-11.2011
Study of novel selective mGlu2 agonist in the temporo-ammonic input to CA1 neurons reveals reduced mGlu2 receptor expression in a Wistar substrain with an anxiety-like phenotype
Abstract
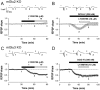
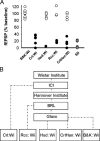
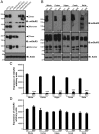
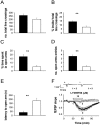
Group II metabotropic receptors (mGluRs) regulate central synaptic transmission by modulating neurotransmitter release. However, the lack of pharmacological tools differentiating between mGlu2 and mGlu3 receptors has hampered identification of the roles of these two receptor subtypes. We have used LY395756 [(1SR,2SR,4RS,5RS,6SR)-2-amino-4-methylbicyclo[3.1.0]-hexane2,6-dicarboxylic], an agonist at mGlu2 receptors and an antagonist at mGlu3 receptors in cell lines, to investigate the roles of these receptors in the temporo-ammonic path from entorhinal cortex to CA1-stratum lacunosum moleculare in rat hippocampal slices. Surprisingly, the degree of inhibition of the field EPSP induced by LY395756 fell into two distinct groups, with EC(50) values of <1 μm and >100 μm. In "sensitive" slices, LY395756 had additive actions with a mixed mGlu2/mGlu3 agonist, DCG-IV [(2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)glycine], whereas in "insensitive" slices, LY395756 reduced the effect of DCG-IV, with an IC(50) of ∼1 μm. This separation into sensitive and insensitive slices could be explained by LY395756 acting as an mGlu2 agonist and mGlu3 antagonist, respectively, a finding supported by data from mice lacking these receptors. The heterogeneity was correlated with differences in expression levels of mGlu2 receptors within our Wistar colony and other Wistar substrains. The initial search for a behavioral correlate indicated that rats lacking mGlu2 receptors showed anxiety-like behavior in open-field and elevated plus maze assays. These findings have implications for rat models of psychiatric disease and are especially pertinent given that mGlu2 receptors are targets for compounds under development for anxiety.
Figures




Similar articles
-
Differentiating the roles of mGlu2 and mGlu3 receptors using LY541850, an mGlu2 agonist/mGlu3 antagonist.Neuropharmacology. 2013 Mar;66:114-21. doi: 10.1016/j.neuropharm.2012.02.023. Epub 2012 Mar 15. Neuropharmacology. 2013. PMID: 22445601
-
Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn.Neuroscience. 2000;100(2):393-406. doi: 10.1016/s0306-4522(00)00269-4. Neuroscience. 2000. PMID: 11008177
-
Selective activation of either mGlu2 or mGlu3 receptors can induce LTD in the amygdala.Neuropharmacology. 2013 Mar;66:196-201. doi: 10.1016/j.neuropharm.2012.04.006. Epub 2012 Apr 17. Neuropharmacology. 2013. PMID: 22531751
-
Impaired expression and function of group II metabotropic glutamate receptors in pilocarpine-treated chronically epileptic rats.Brain Res. 2008 Nov 13;1240:165-76. doi: 10.1016/j.brainres.2008.08.084. Epub 2008 Sep 10. Brain Res. 2008. PMID: 18804094 Free PMC article.
-
Prevalence and influence of cys407* Grm2 mutation in Hannover-derived Wistar rats: mGlu2 receptor loss links to alcohol intake, risk taking and emotional behaviour.Neuropharmacology. 2017 Mar 15;115:128-138. doi: 10.1016/j.neuropharm.2016.03.020. Epub 2016 Mar 14. Neuropharmacology. 2017. PMID: 26987983 Review.
Cited by
-
Persistent nociception induces anxiety-like behavior in rodents: role of endogenous neuropeptide S.Pain. 2014 Aug;155(8):1504-1515. doi: 10.1016/j.pain.2014.04.026. Epub 2014 May 2. Pain. 2014. PMID: 24793908 Free PMC article.
-
Genetic deletion of mGlu2 metabotropic glutamate receptors improves the short-term outcome of cerebral transient focal ischemia.Mol Brain. 2017 Aug 18;10(1):39. doi: 10.1186/s13041-017-0319-6. Mol Brain. 2017. PMID: 28821279 Free PMC article.
-
In vitro characterization of cell-level neurophysiological diversity in the rostral nucleus reuniens of adult mice.J Physiol. 2017 Jun 1;595(11):3549-3572. doi: 10.1113/JP273915. Epub 2017 Apr 25. J Physiol. 2017. PMID: 28295330 Free PMC article.
-
LY395756, an mGluR2 agonist and mGluR3 antagonist, enhances NMDA receptor expression and function in the normal adult rat prefrontal cortex, but fails to improve working memory and reverse MK801-induced working memory impairment.Exp Neurol. 2015 Nov;273:190-201. doi: 10.1016/j.expneurol.2015.08.019. Epub 2015 Sep 1. Exp Neurol. 2015. PMID: 26341392 Free PMC article.
-
The role of group II metabotropic glutamate receptors in cognition and anxiety: comparative studies in GRM2(-/-), GRM3(-/-) and GRM2/3(-/-) knockout mice.Neuropharmacology. 2015 Feb;89:19-32. doi: 10.1016/j.neuropharm.2014.08.010. Epub 2014 Aug 23. Neuropharmacology. 2015. PMID: 25158312 Free PMC article.
References
-
- Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods. 2007;162:346–356. - PubMed
-
- Bond A, Monn JA, Lodge D. A novel orally active group 2 metabotropic glutamate receptor agonist: LY354740. Neuroreport. 1997;8:1463–1466. - PubMed
-
- Bond A, O'Neill MJ, Hicks CA, Monn JA, Lodge D. Neuroprotective effects of a systemically active group II metabotropic glutamate receptor agonist LY354740 in a gerbil model of global ischaemia. Neuroreport. 1998;9:1191–1193. - PubMed
-
- Bruno V, Battaglia G, Copani A, D'Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab. 2001;21:1013–1033. - PubMed
-
- Capogna M. Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway-CA1 EPSCs. Eur J Neurosci. 2004;19:2847–2858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
