BACE1 activity is modulated by cell-associated sphingosine-1-phosphate
- PMID: 21543615
- PMCID: PMC4534000
- DOI: 10.1523/JNEUROSCI.6467-10.2011
BACE1 activity is modulated by cell-associated sphingosine-1-phosphate
Abstract
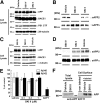
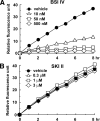
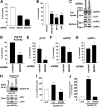
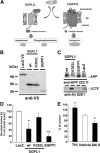
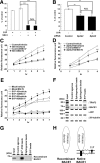
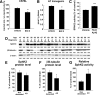
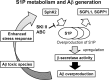
Sphingosine kinase (SphK) 1 and 2 phosphorylate sphingosine to generate sphingosine-1-phosphate (S1P), a pluripotent lipophilic mediator implicated in a variety of cellular events. Here we show that the activity of β-site APP cleaving enzyme-1 (BACE1), the rate-limiting enzyme for amyloid-β peptide (Aβ) production, is modulated by S1P in mouse neurons. Treatment by SphK inhibitor, RNA interference knockdown of SphK, or overexpression of S1P degrading enzymes decreased BACE1 activity, which reduced Aβ production. S1P specifically bound to full-length BACE1 and increased its proteolytic activity, suggesting that cellular S1P directly modulates BACE1 activity. Notably, the relative activity of SphK2 was upregulated in the brains of patients with Alzheimer's disease. The unique modulatory effect of cellular S1P on BACE1 activity is a novel potential therapeutic target for Alzheimer's disease.
Figures

Similar articles
-
Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article.
-
Relationship between ubiquilin-1 and BACE1 in human Alzheimer's disease and APdE9 transgenic mouse brain and cell-based models.Neurobiol Dis. 2016 Jan;85:187-205. doi: 10.1016/j.nbd.2015.11.005. Epub 2015 Nov 10. Neurobiol Dis. 2016. PMID: 26563932
-
SEPT8 modulates β-amyloidogenic processing of APP by affecting the sorting and accumulation of BACE1.J Cell Sci. 2016 Jun 1;129(11):2224-38. doi: 10.1242/jcs.185215. Epub 2016 Apr 15. J Cell Sci. 2016. PMID: 27084579
-
The β-Secretase Enzyme BACE1: A Biochemical Enigma for Alzheimer's Disease.CNS Neurol Disord Drug Targets. 2020;19(3):184-194. doi: 10.2174/1871527319666200526144141. CNS Neurol Disord Drug Targets. 2020. PMID: 32452328 Review.
-
BACE1: the beta-secretase enzyme in Alzheimer's disease.J Mol Neurosci. 2004;23(1-2):105-14. doi: 10.1385/JMN:23:1-2:105. J Mol Neurosci. 2004. PMID: 15126696 Review.
Cited by
-
Cross-talk of membrane lipids and Alzheimer-related proteins.Mol Neurodegener. 2013 Oct 22;8:34. doi: 10.1186/1750-1326-8-34. Mol Neurodegener. 2013. PMID: 24148205 Free PMC article. Review.
-
The impact of cholesterol, DHA, and sphingolipids on Alzheimer's disease.Biomed Res Int. 2013;2013:814390. doi: 10.1155/2013/814390. Epub 2014 Feb 19. Biomed Res Int. 2013. PMID: 24575399 Free PMC article. Review.
-
Role of sphingolipid metabolism in neurodegeneration.J Neurochem. 2021 Jul;158(1):25-35. doi: 10.1111/jnc.15044. Epub 2020 Jul 3. J Neurochem. 2021. PMID: 32402091 Free PMC article. Review.
-
Sphingolipid Metabolism: A New Therapeutic Opportunity for Brain Degenerative Disorders.Front Neurosci. 2018 Apr 17;12:249. doi: 10.3389/fnins.2018.00249. eCollection 2018. Front Neurosci. 2018. PMID: 29719499 Free PMC article. Review.
-
Recent Insight into the Role of Sphingosine-1-Phosphate Lyase in Neurodegeneration.Int J Mol Sci. 2023 Mar 24;24(7):6180. doi: 10.3390/ijms24076180. Int J Mol Sci. 2023. PMID: 37047151 Free PMC article. Review.
References
-
- Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
