Gut sensing of dietary K⁺ intake increases renal K⁺excretion
- PMID: 21543632
- PMCID: PMC3154709
- DOI: 10.1152/ajpregu.00095.2011
Gut sensing of dietary K⁺ intake increases renal K⁺excretion
Abstract
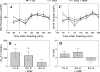
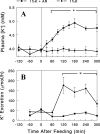
Dietary K(+) intake may increase renal K(+) excretion via increasing plasma [K(+)] and/or activating a mechanism independent of plasma [K(+)]. We evaluated these mechanisms during normal dietary K(+) intake. After an overnight fast, [K(+)] and renal K(+) excretion were measured in rats fed either 0% K(+) or the normal 1% K(+) diet. In a third group, rats were fed with the 0% K(+) diet, and KCl was infused to match plasma [K(+)] profile to that of the 1% K(+) diet group. The 1% K(+) feeding significantly increased renal K(+) excretion, associated with slight increases in plasma [K(+)], whereas the 0% K(+) diet decreased K(+) excretion, associated with decreases in plasma [K(+)]. In the KCl-infused 0% K(+) diet group, renal K(+) excretion was significantly less than that of the 1% K(+) group, despite matched plasma [K(+)] profiles. We also examined whether dietary K(+) alters plasma profiles of gut peptides, such as guanylin, uroguanylin, glucagon-like peptide 1, and glucose-dependent insulinotropic polypeptide, pituitary peptides, such as AVP, α-MSH, and γ-MSH, or aldosterone. Our data do not support a role for these hormones in the stimulation of renal K(+) excretion during normal K(+) intake. In conclusion, postprandial increases in renal K(+) excretion cannot be fully accounted for by changes in plasma [K(+)] and that gut sensing of dietary K(+) is an important component of the regulation of renal K(+) excretion. Our studies on gut and pituitary peptide hormones suggest that there may be previously unknown humoral factors that stimulate renal K(+) excretion during dietary K(+) intake.
Figures






References
-
- Ahren B. GLP-1 and extra-islet effects. Horm Metab Res 36: 842–845, 2004 - PubMed
-
- Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am J Physiol Renal Fluid Electrolyte Physiol 240: F257–F268, 1981 - PubMed
-
- Calo L, Borsatti A, Favaro S, Rabinowitz L. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron 69: 253–258, 1995 - PubMed
-
- Choi CS, Lee FN, McDonough AA, Youn JH. Independent regulation of in vivo insulin action on glucose versus K+ uptake by dietary fat and K+ content. Diabetes 51: 915–920, 2002 - PubMed
-
- Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH. Short-term K+ deprivation provokes insulin resistance of cellular K+ uptake revealed with the K+ clamp. Am J Physiol Renal Physiol 280: F95–F102, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

