The Ia.2 epitope defines a subset of lipid raft-resident MHC class II molecules crucial to effective antigen presentation
- PMID: 21543648
- PMCID: PMC4860613
- DOI: 10.4049/jimmunol.1100336
The Ia.2 epitope defines a subset of lipid raft-resident MHC class II molecules crucial to effective antigen presentation
Abstract
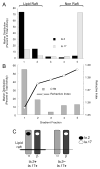
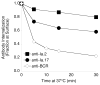
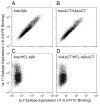
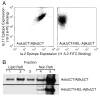
Previous work established that binding of the 11-5.2 anti-I-A(k) mAb, which recognizes the Ia.2 epitope on I-A(k) class II molecules, elicits MHC class II signaling, whereas binding of two other anti-I-A(k) mAbs that recognize the Ia.17 epitope fail to elicit signaling. Using a biochemical approach, we establish that the Ia.2 epitope recognized by the widely used 11-5.2 mAb defines a subset of cell surface I-A(k) molecules predominantly found within membrane lipid rafts. Functional studies demonstrate that the Ia.2-bearing subset of I-A(k) class II molecules is critically necessary for effective B cell-T cell interactions, especially at low Ag doses, a finding consistent with published studies on the role of raft-resident class II molecules in CD4 T cell activation. Interestingly, B cells expressing recombinant I-A(k) class II molecules possessing a β-chain-tethered hen egg lysosome peptide lack the Ia.2 epitope and fail to partition into lipid rafts. Moreover, cells expressing Ia.2(-) tethered peptide-class II molecules are severely impaired in their ability to present both tethered peptide or peptide derived from exogenous Ag to CD4 T cells. These results establish the Ia.2 epitope as defining a lipid raft-resident MHC class II conformer vital to the initiation of MHC class II-restricted B cell-T cell interactions.
Figures


Similar articles
-
Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation.Nat Immunol. 2000 Aug;1(2):156-62. doi: 10.1038/77842. Nat Immunol. 2000. PMID: 11248809
-
MHC class II-peptide complexes and APC lipid rafts accumulate at the immunological synapse.J Immunol. 2003 Feb 1;170(3):1329-38. doi: 10.4049/jimmunol.170.3.1329. J Immunol. 2003. PMID: 12538693
-
Contrasting cytoskeletal regulation of MHC class II peptide presentation by human B cells or dendritic cells.Eur J Immunol. 2008 Apr;38(4):1096-105. doi: 10.1002/eji.200737455. Eur J Immunol. 2008. PMID: 18393378
-
Association of MHC class II-peptide complexes with plasma membrane lipid microdomains.Curr Opin Immunol. 2004 Feb;16(1):103-7. doi: 10.1016/j.coi.2003.11.009. Curr Opin Immunol. 2004. PMID: 14734117 Review.
-
MHC class II association with lipid rafts on the antigen presenting cell surface.Biochim Biophys Acta. 2015 Apr;1853(4):775-80. doi: 10.1016/j.bbamcr.2014.09.019. Epub 2014 Sep 28. Biochim Biophys Acta. 2015. PMID: 25261705 Free PMC article. Review.
Cited by
-
Differential transmembrane domain GXXXG motif pairing impacts major histocompatibility complex (MHC) class II structure.J Biol Chem. 2014 Apr 25;289(17):11695-11703. doi: 10.1074/jbc.M113.516997. Epub 2014 Mar 11. J Biol Chem. 2014. PMID: 24619409 Free PMC article.
-
The immunobiology of ubiquitin-dependent B cell receptor functions.Mol Immunol. 2018 Sep;101:146-154. doi: 10.1016/j.molimm.2018.05.022. Epub 2018 Jun 26. Mol Immunol. 2018. PMID: 29940407 Free PMC article. Review.
-
Immunological Functions of the Membrane Proximal Region of MHC Class II Molecules.F1000Res. 2016 Mar 17;5:F1000 Faculty Rev-368. doi: 10.12688/f1000research.7610.1. eCollection 2016. F1000Res. 2016. PMID: 27006762 Free PMC article. Review.
-
Targeting Antigens to CD180 but Not CD40 Programs Immature and Mature B Cell Subsets to Become Efficient APCs.J Immunol. 2019 Oct 1;203(7):1715-1729. doi: 10.4049/jimmunol.1900549. Epub 2019 Sep 4. J Immunol. 2019. PMID: 31484732 Free PMC article.
-
A triad of molecular regions contribute to the formation of two distinct MHC class II conformers.Mol Immunol. 2016 Jun;74:59-70. doi: 10.1016/j.molimm.2016.04.010. Epub 2016 May 2. Mol Immunol. 2016. PMID: 27148821 Free PMC article.
References
-
- Sadegh-Nasseri S, Stern LJ, Wiley DC, Germain RN. MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature. 1994;370:647–650. - PubMed
-
- Lovitch SB, Unanue ER. Conformational isomers of a peptide-class II major histocompatibility complex. Immunol Rev. 2005;207:293–313. - PubMed
-
- Klein J. The major histocompatibility complex of the mouse. Science. 1979;203:516–521. - PubMed
-
- Oi VT, Jones PP, Goding JW, Herzenberg LA. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. - PubMed
-
- Pierres M, Devaux C, Dosseto M, Marchetto S. Clonal analysis of B- and T-cell responses to Ia antigens. I. Topology of epitope regions on I-Ak and I-Ek molecules analyzed with 35 monoclonal alloantibodies. Immunogenetics. 1981;14:481–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

