Water molecule reorganization in cytochrome c oxidase revealed by FTIR spectroscopy
- PMID: 21543712
- PMCID: PMC3102355
- DOI: 10.1073/pnas.1019419108
Water molecule reorganization in cytochrome c oxidase revealed by FTIR spectroscopy
Abstract
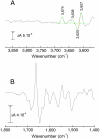
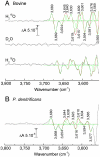
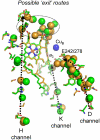
Although internal electron transfer and oxygen reduction chemistry in cytochrome c oxidase are fairly well understood, the associated groups and pathways that couple these processes to gated proton translocation across the membrane remain unclear. Several possible pathways have been identified from crystallographic structural models; these involve hydrophilic residues in combination with structured waters that might reorganize to form transient proton transfer pathways during the catalytic cycle. To date, however, comparisons of atomic structures of different oxidases in different redox or ligation states have not provided a consistent answer as to which pathways are operative or the details of their dynamic changes during catalysis. In order to provide an experimental means to address this issue, FTIR spectroscopy in the 3,560-3,800 cm(-1) range has been used to detect weakly H-bonded water molecules in bovine cytochrome c oxidase that might change during catalysis. Full redox spectra exhibited at least four signals at 3,674(+), 3,638(+), 3,620(-), and 3,607(+) cm(-1). A more complex set of signals was observed in spectra of photolysis of the ferrous-CO compound, a reaction that mimics the catalytic oxygen binding step, and their D(2)O and H(2)(18)O sensitivities confirmed that they arose from water molecule rearrangements. Fitting with Gaussian components indicated the involvement of up to eight waters in the photolysis transition. Similar signals were also observed in photolysis spectra of the ferrous-CO compound of bacterial CcO from Paracoccus denitrificans. Such water changes are discussed in relation to roles in hydrophilic channels and proton/electron coupling mechanism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rich PR, Maréchal A. The mitochondrial respiratory chain. Essays Biochem. 2010;27:1–23. - PubMed
-
- Kaila VRI, Verkhovsky MI, Wikström M. Proton-coupled electron transfer in cytochrome oxidase. Chem Rev. 2010;110:7062–7081. - PubMed
-
- Tsukihara T, et al. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science. 1996;272:1136–1144. - PubMed
-
- Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 Å resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

