Unforeseen decreases in dissolved oxygen levels affect tube formation kinetics in collagen gels
- PMID: 21543738
- PMCID: PMC3154554
- DOI: 10.1152/ajpcell.00074.2011
Unforeseen decreases in dissolved oxygen levels affect tube formation kinetics in collagen gels
Abstract
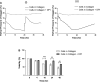
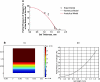
The availability of oxygen (O(2)) is a critical parameter affecting vascular tube formation. In this study, we hypothesize that dissolved oxygen (DO) levels in collagen gels change during the three-dimensional (3D) culture of human umbilical vein endothelial cells (HUVECs) in atmospheric conditions and that such changes affect the kinetics of tube formation through the production of reactive oxygen species (ROS). We demonstrate a decrease in O(2) tension during 3D cultures of HUVECs. Noninvasive measurements of DO levels during culture under atmospheric conditions revealed a profound decrease that reached as low as 2% O(2) at the end of 24 h. After media replacement, DO levels rose rapidly and equilibrated at ∼15% O(2), creating a reoxygenated environment. To accurately estimate DO gradients in 3D collagen gels, we developed a 3D mathematical model and determined the Michaelis-Menten parameters, V(max) and K(m), of HUVECs in collagen gels. We detected an increase in ROS levels throughout the culture period. Using diphenyliodonium to inhibit ROS production resulted in the complete inhibition of tube formation. Interference RNA studies further showed that hypoxia-inducible factors (HIFs)-1α and -2α are not involved in the formation of 3D tubes in collagen gels. We conclude that ROS affect the tubulogenesis process through HIFα-independent pathways, where the levels of ROS are influenced by the uncontrolled variations in DO levels. This study is the first demonstration of the critical and unexpected role of O(2) during 3D in vitro culture models of tubulogenesis in atmospheric conditions.
Figures



References
-
- Abid MR, Kachra Z, Spokes KC, Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett 486: 252–256, 2000 - PubMed
-
- Akimoto T, Liapis H, Hammerman MR. Microvessel formation from mouse embryonic aortic explants is oxygen and VEGF dependent. Am J Physiol Regul Integr Comp Physiol 283: R487–R495, 2002 - PubMed
-
- Ben-Yosef Y, Miller A, Shapiro S, Lahat N. Hypoxia of endothelial cells leads to MMP-2-dependent survival and death. Am J Physiol Cell Physiol 289: C1321–C1331, 2005 - PubMed
-
- Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem 282: 8019–8026, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

